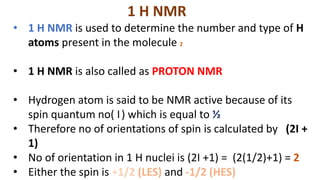
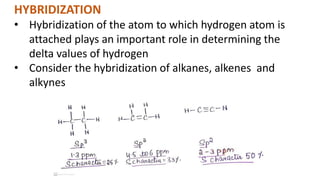
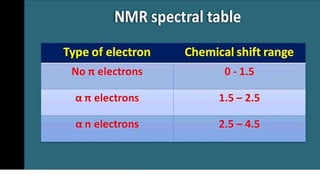
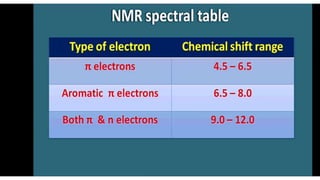
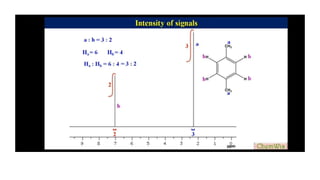
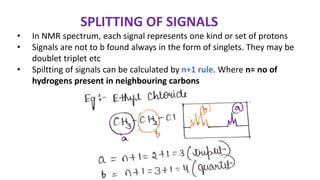
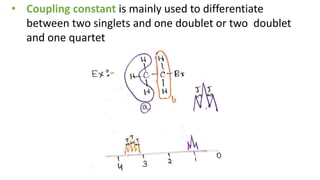
The document provides an overview of 1H NMR spectroscopy, detailing its principles, mechanisms, and applications. It explains the process of nuclear magnetic resonance, including how spinning nuclei interact with external magnetic fields, the significance of chemical shifts, and methods for integrating spectral data. Additionally, it covers important factors affecting chemical shifts and the role of spin-spin coupling in determining spectral signals.