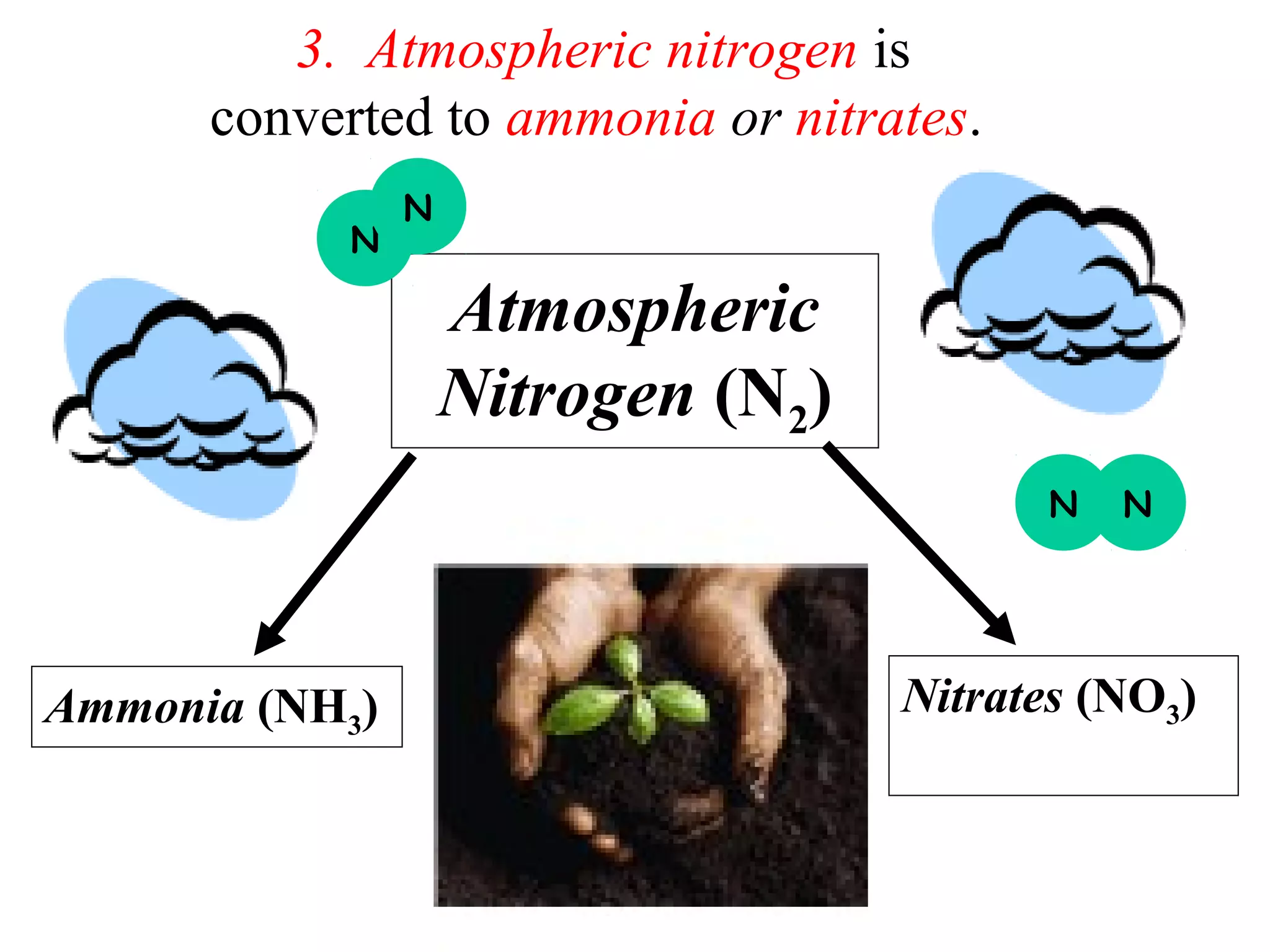
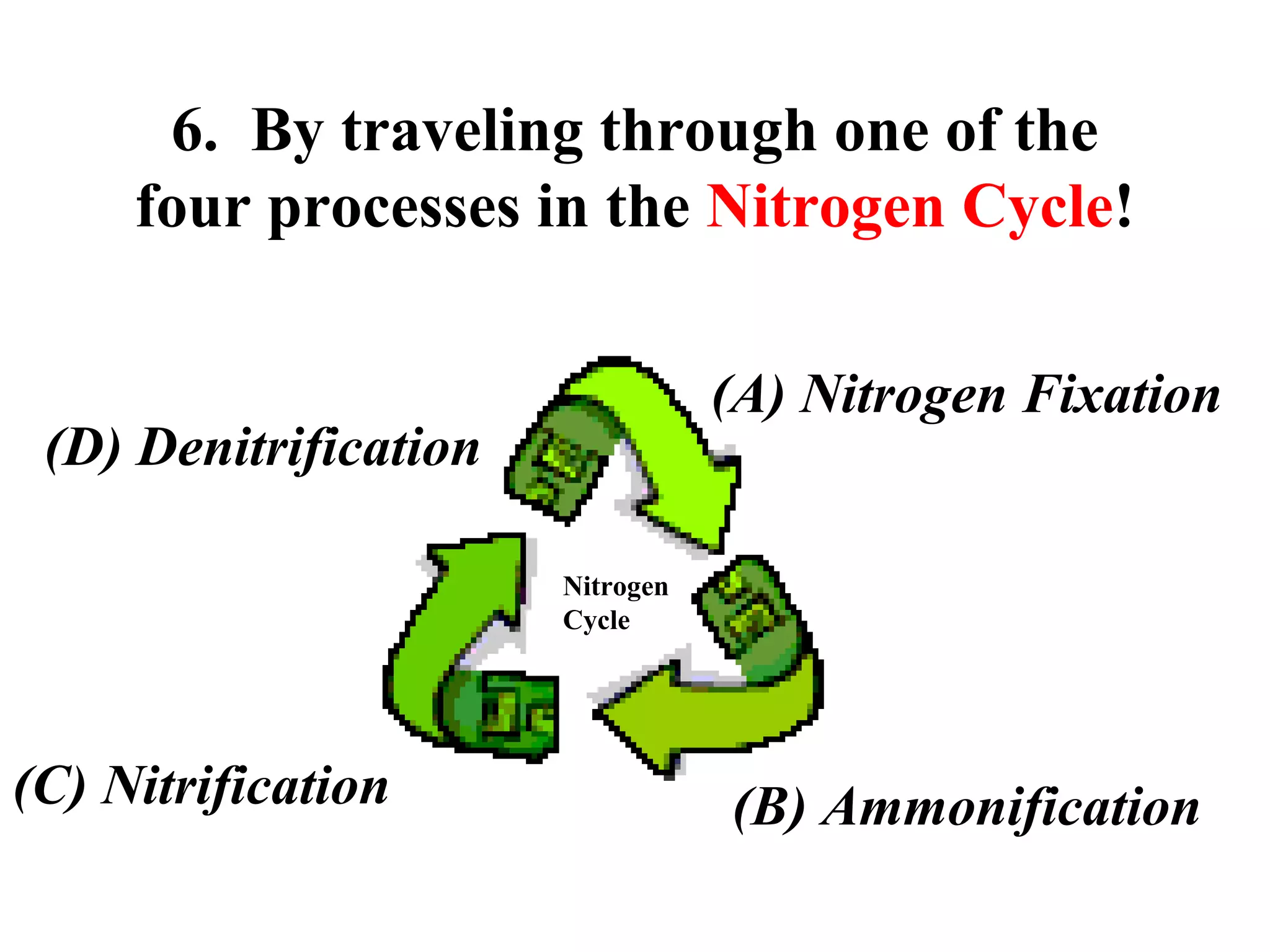
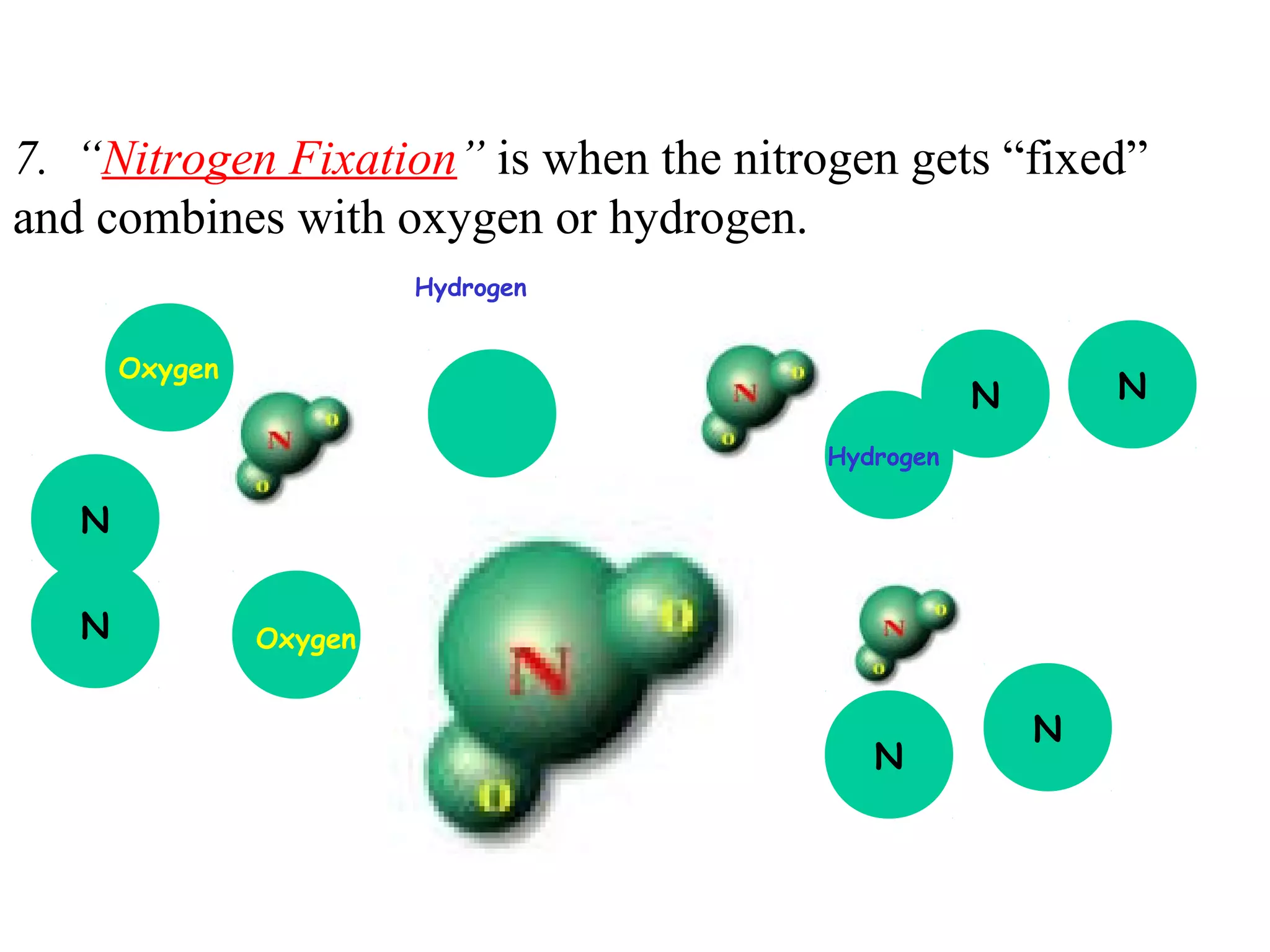
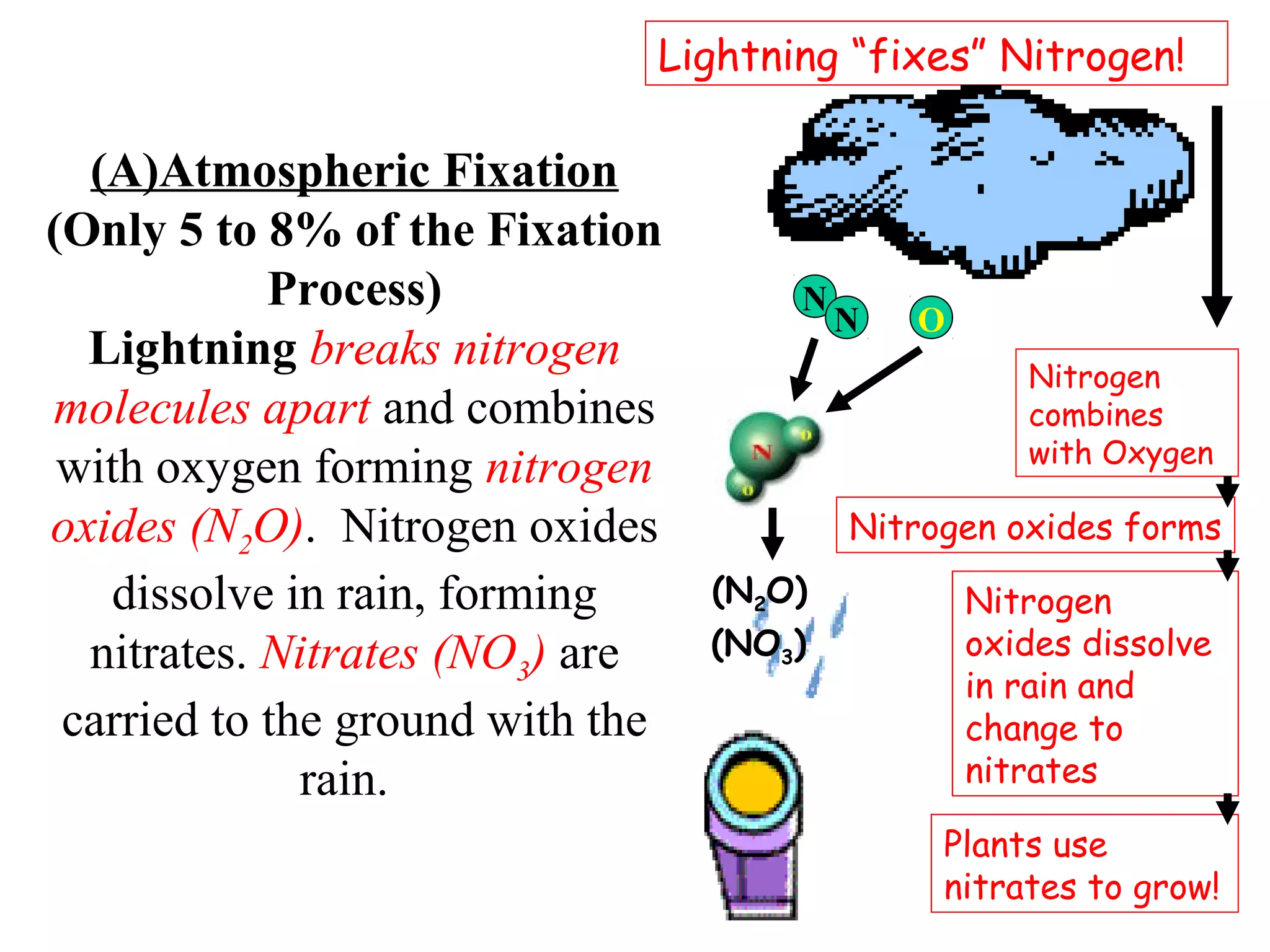
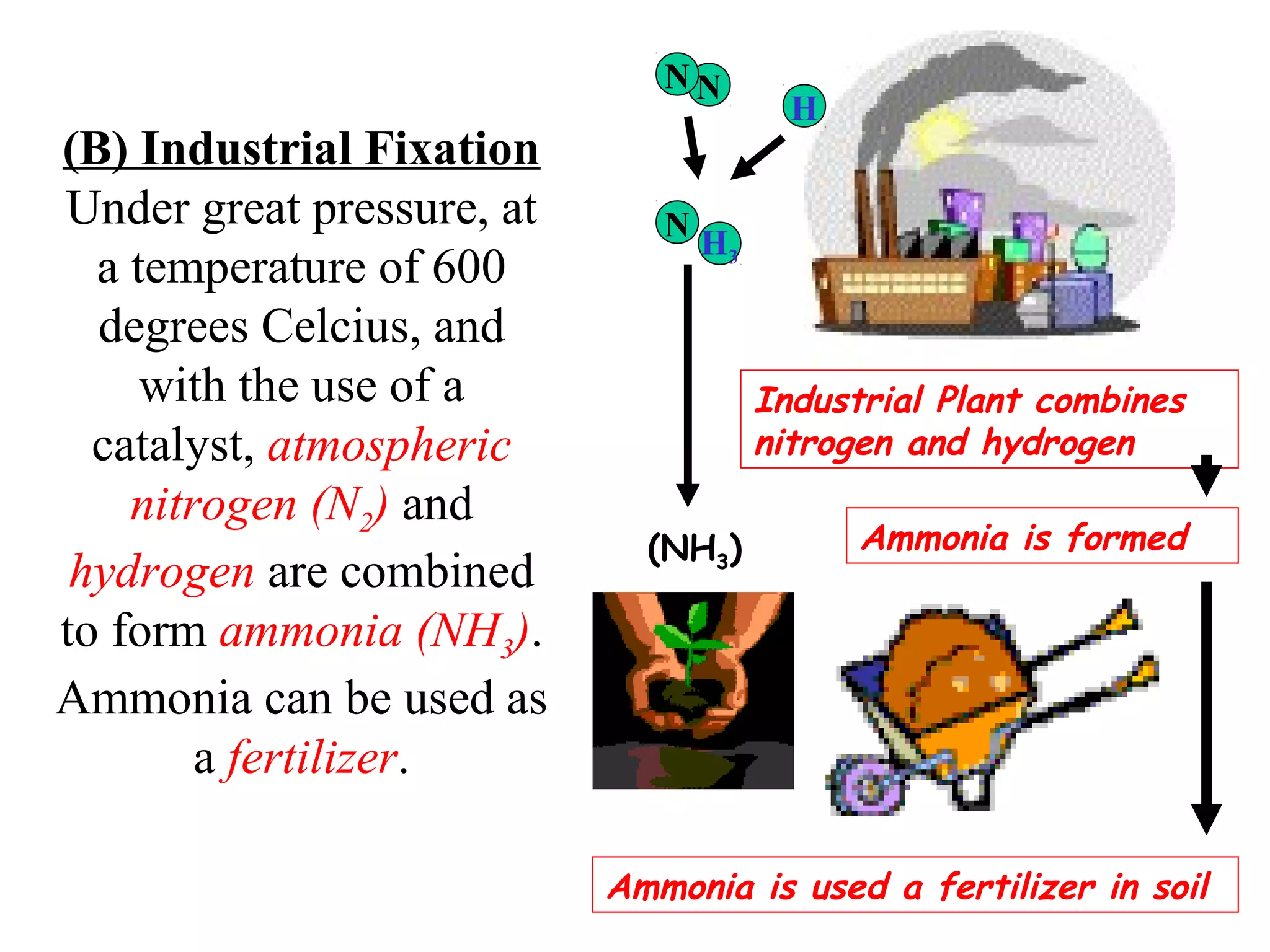
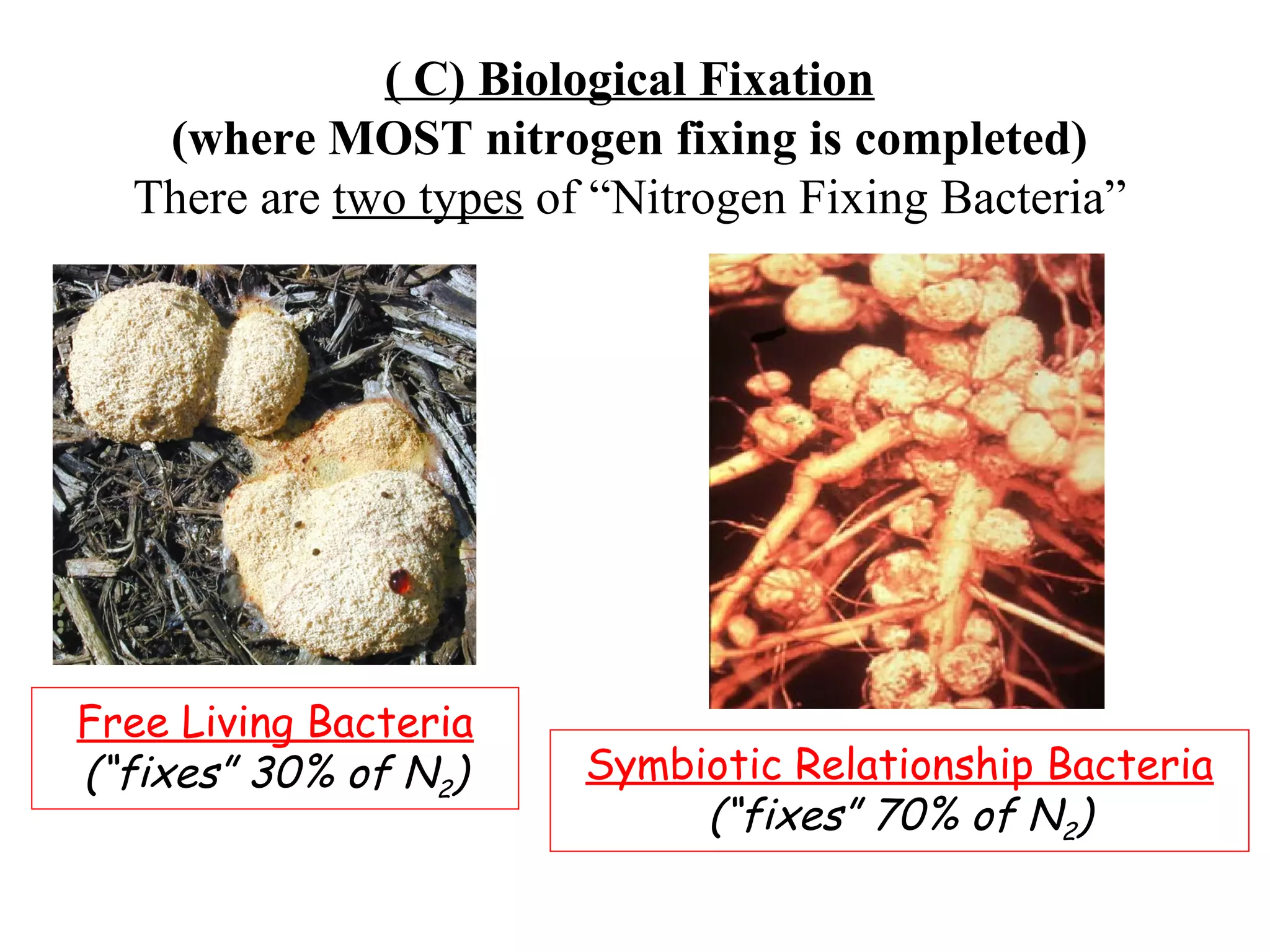
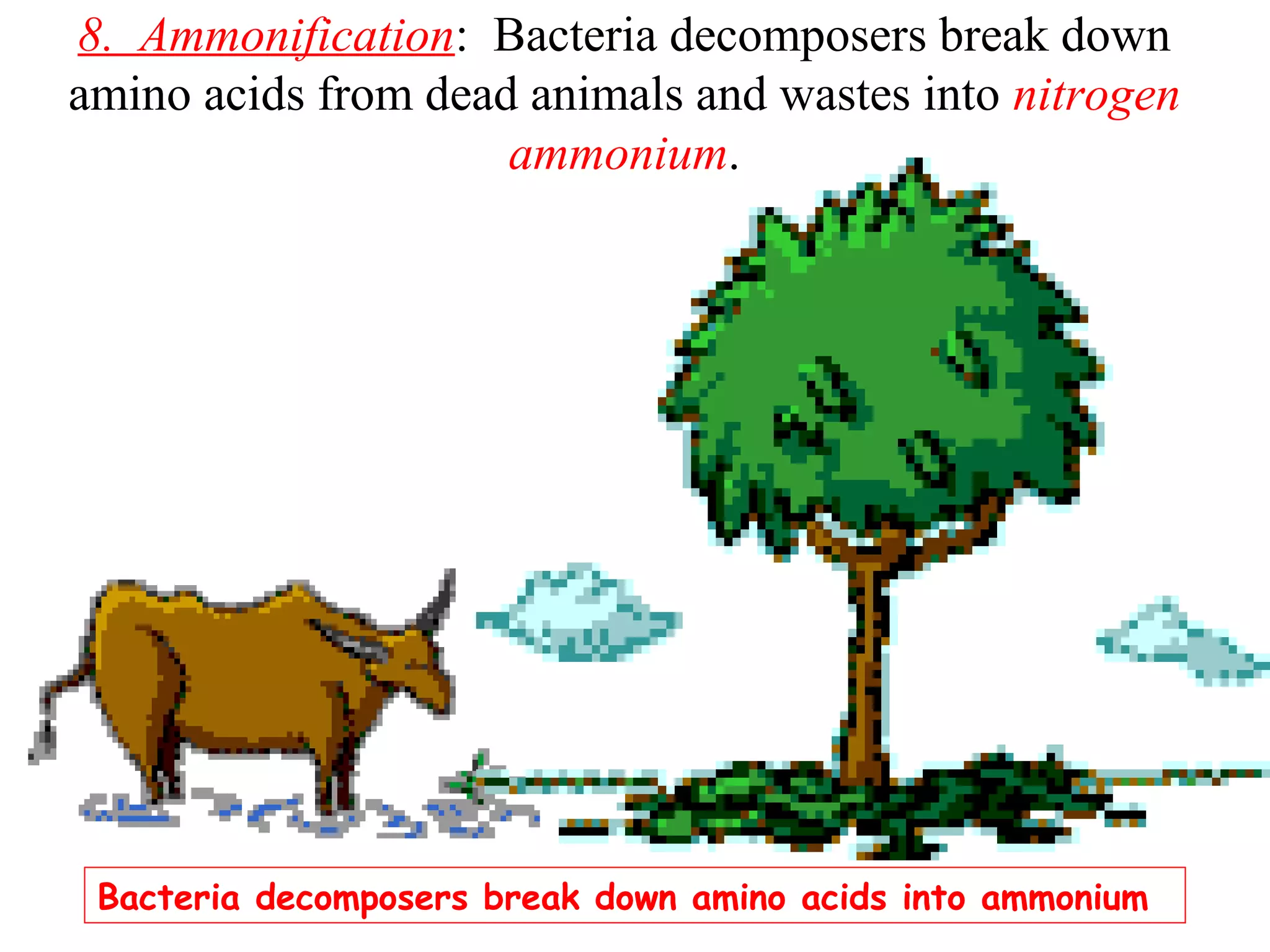
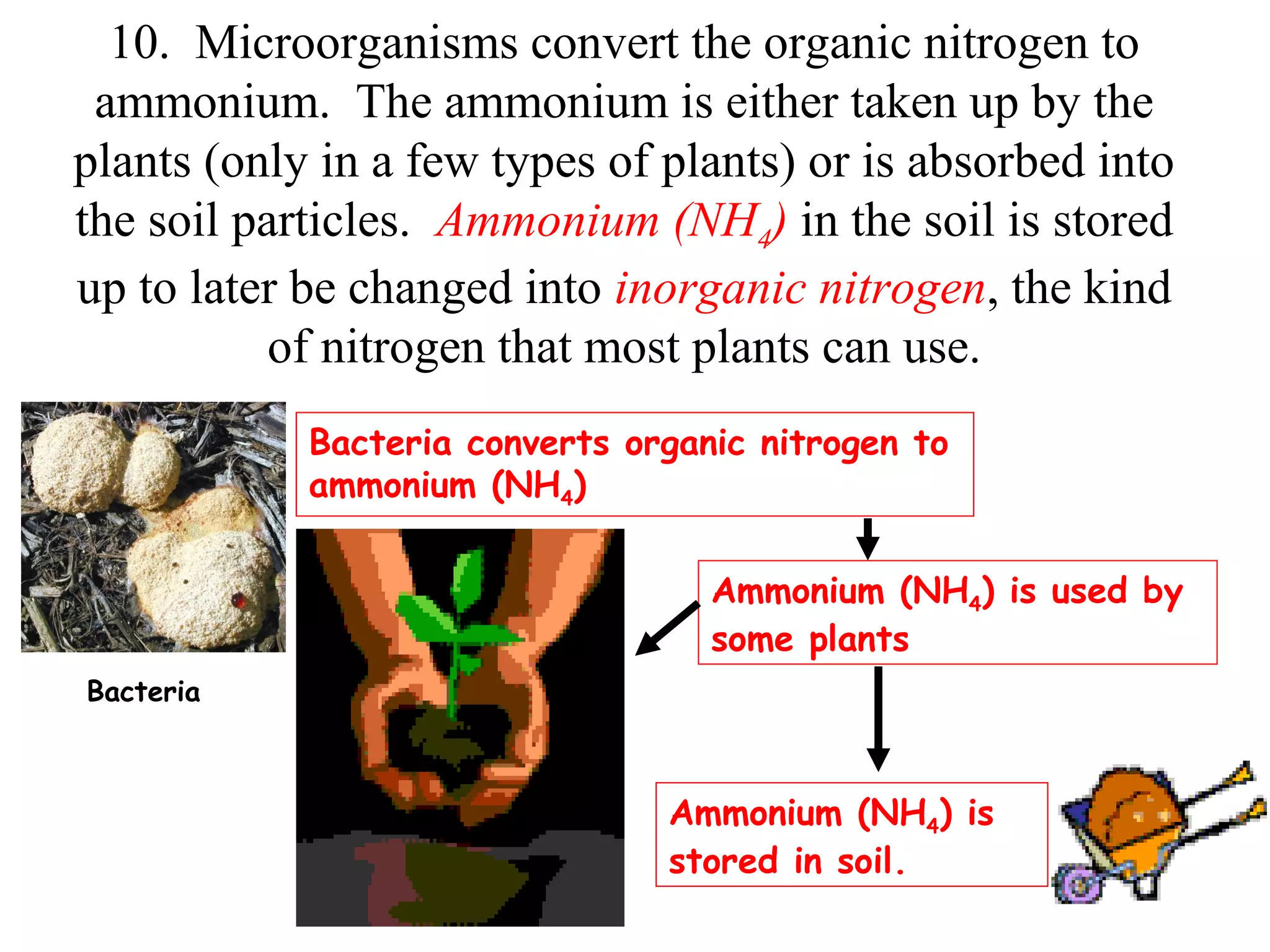
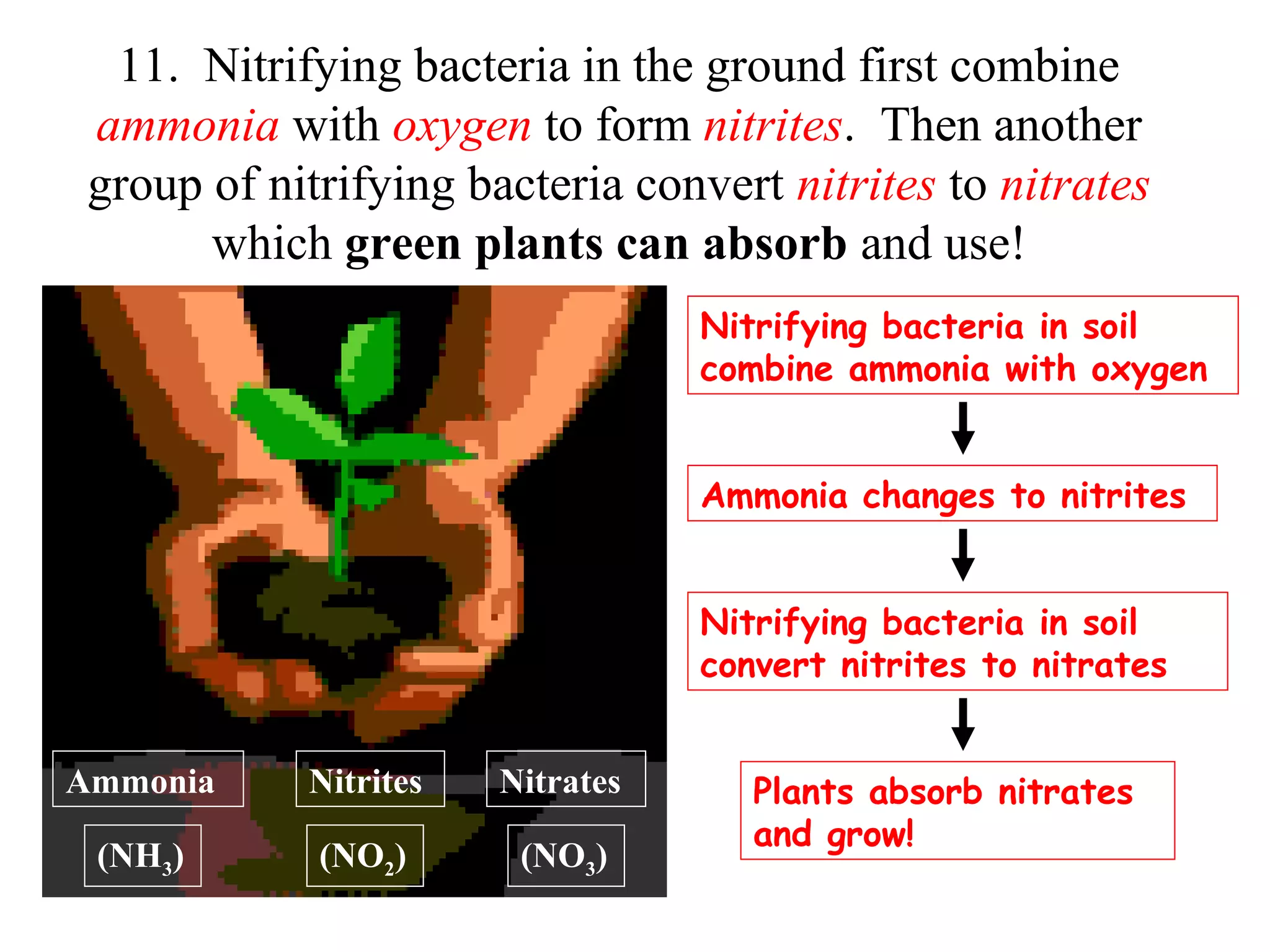
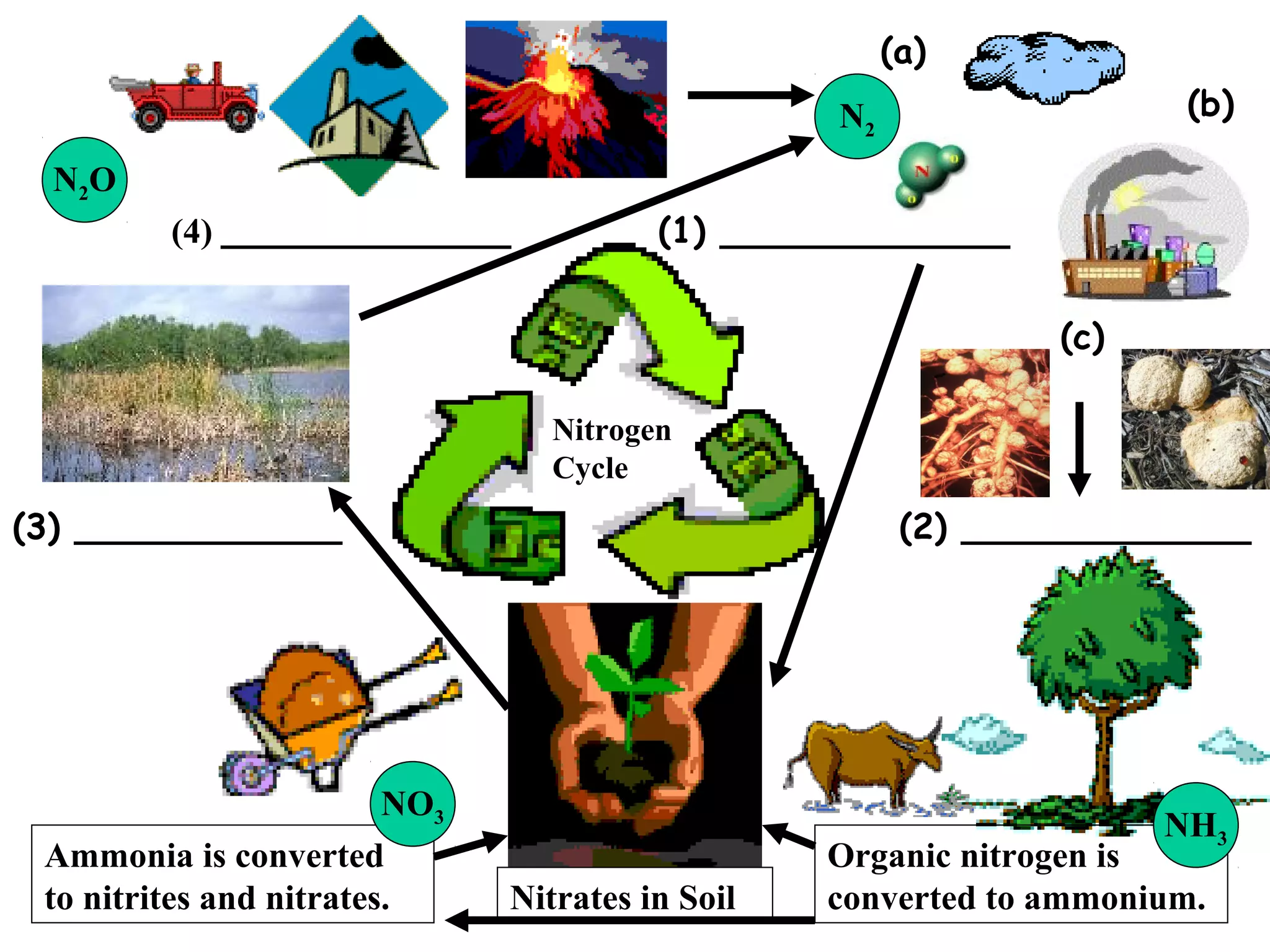
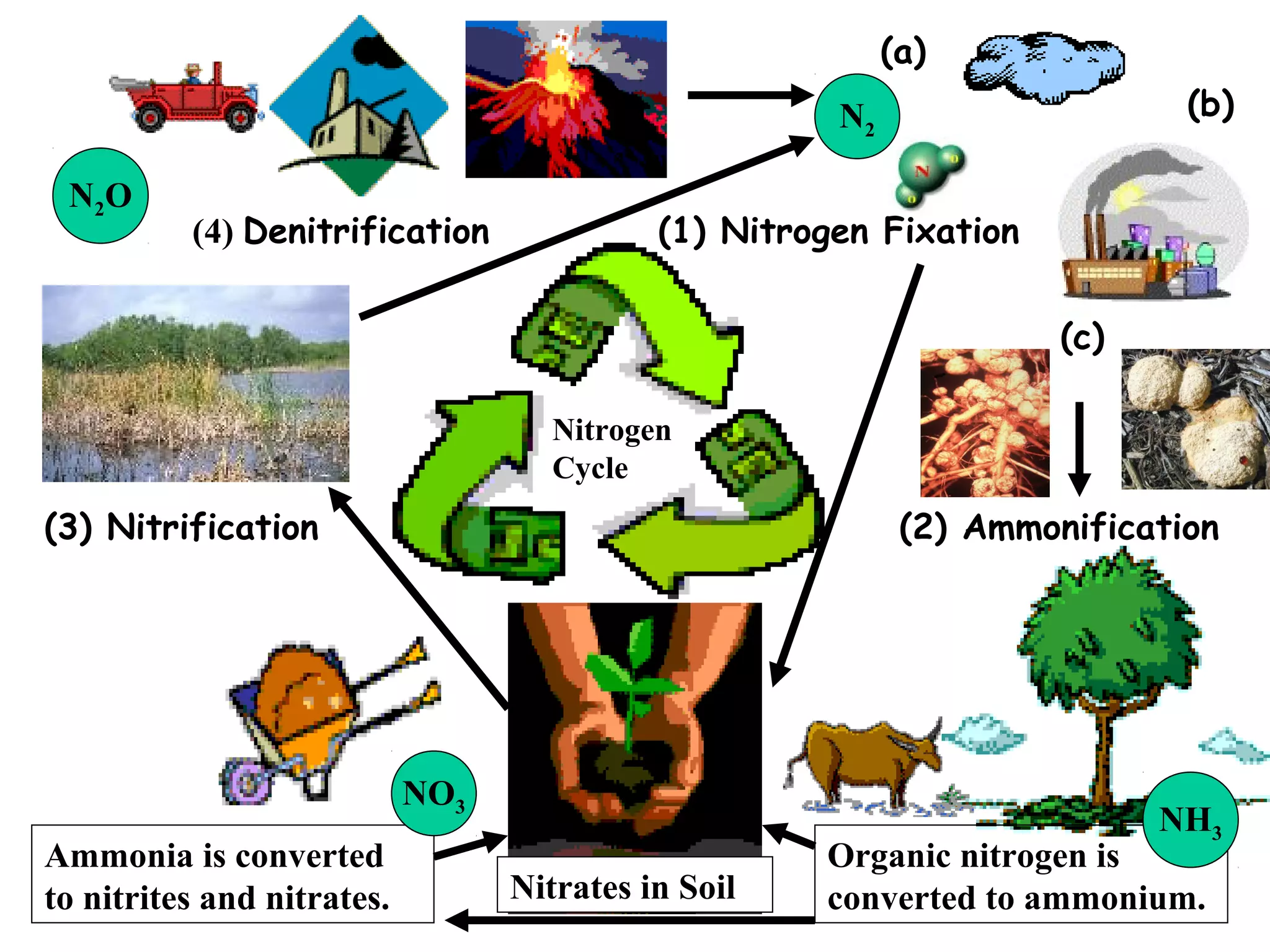
The nitrogen cycle describes how nitrogen is converted between its various forms as it circulates in the biosphere and lithosphere. Atmospheric nitrogen is converted to ammonia or nitrates through nitrogen fixation, which occurs via lightning, industrial fixation, or biological fixation by bacteria. Ammonia is converted to nitrites and nitrates through nitrification by nitrifying bacteria. Organic nitrogen from dead organisms is broken down into ammonium through ammonification by bacteria. Nitrates are converted back to atmospheric nitrogen through denitrification, replenishing the nitrogen in the air.