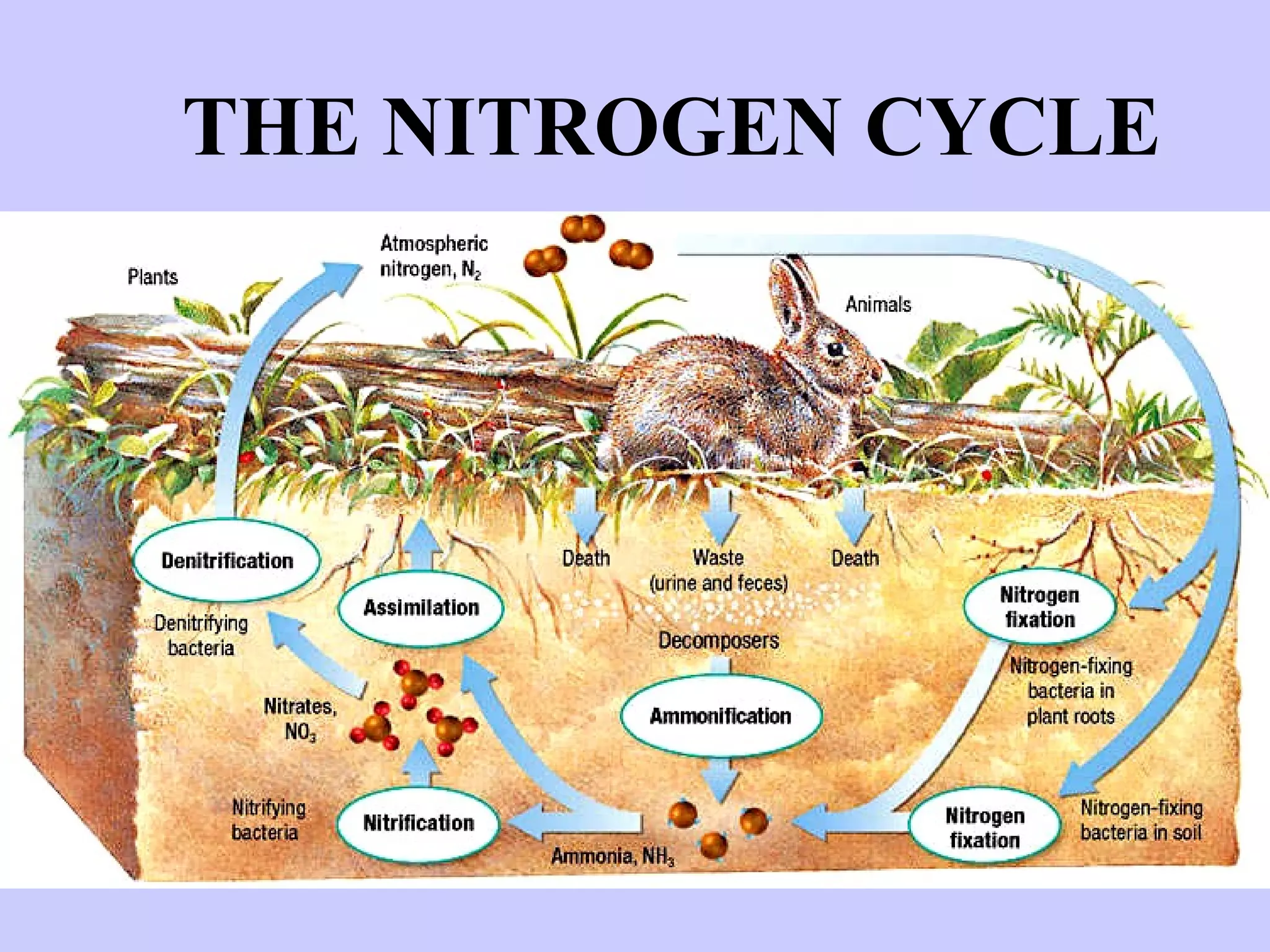
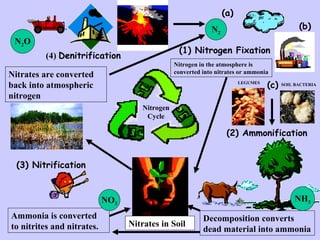
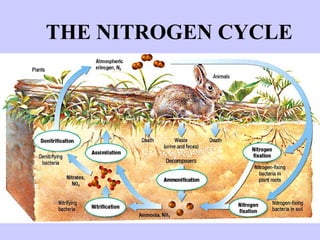
1. The nitrogen cycle describes how nitrogen is converted between its various forms and moves between the atmosphere, soil, plants, and animals.
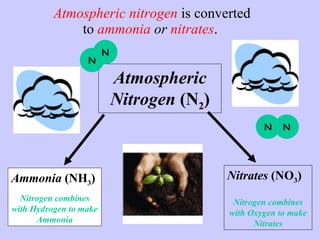
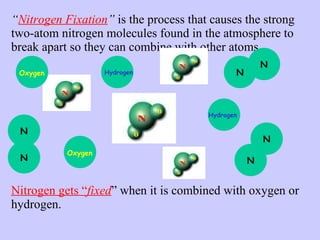
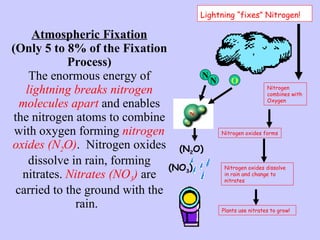
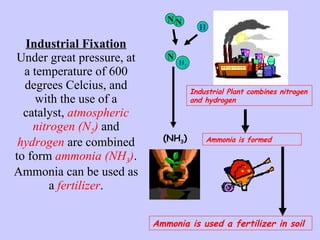
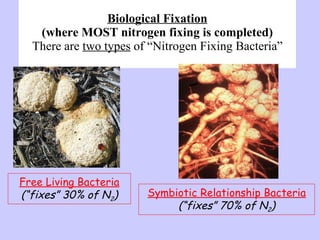
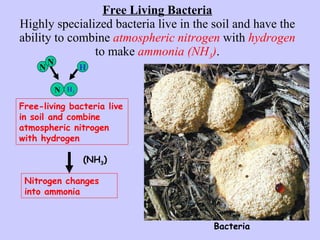
2. Atmospheric nitrogen is converted to ammonia or nitrates through nitrogen fixation, which allows plants and animals to use it.
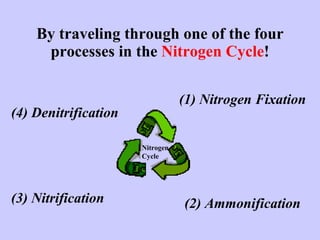
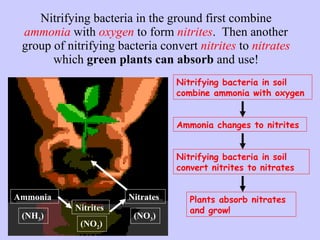
3. Nitrification and ammonification convert ammonia into other nitrogen forms that plants can use, and denitrification returns nitrogen to the atmosphere, completing the cycle.