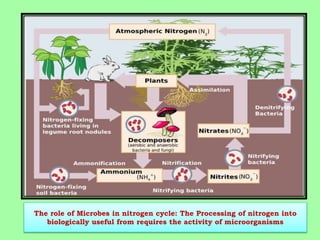
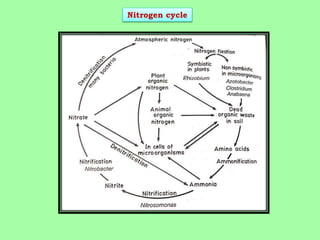
Microbes play a key role in biogeochemical cycles by facilitating the conversion of elements like nitrogen into biologically useful forms. The nitrogen cycle involves nitrogen fixation by bacteria, assimilation by plants, ammonification by microbes breaking down organic matter, nitrification of ammonia to nitrites and nitrates by other microbes, denitrification of nitrates back to nitrogen gas, and sedimentation of nitrogen compounds. Nitrogen is fixed from the atmosphere by lightning, industrial processes, and symbiotic and free-living bacteria, and it is then incorporated into living tissues through assimilation or converted between forms like ammonium, nitrite and nitrate by a variety of microorganisms in the soil.