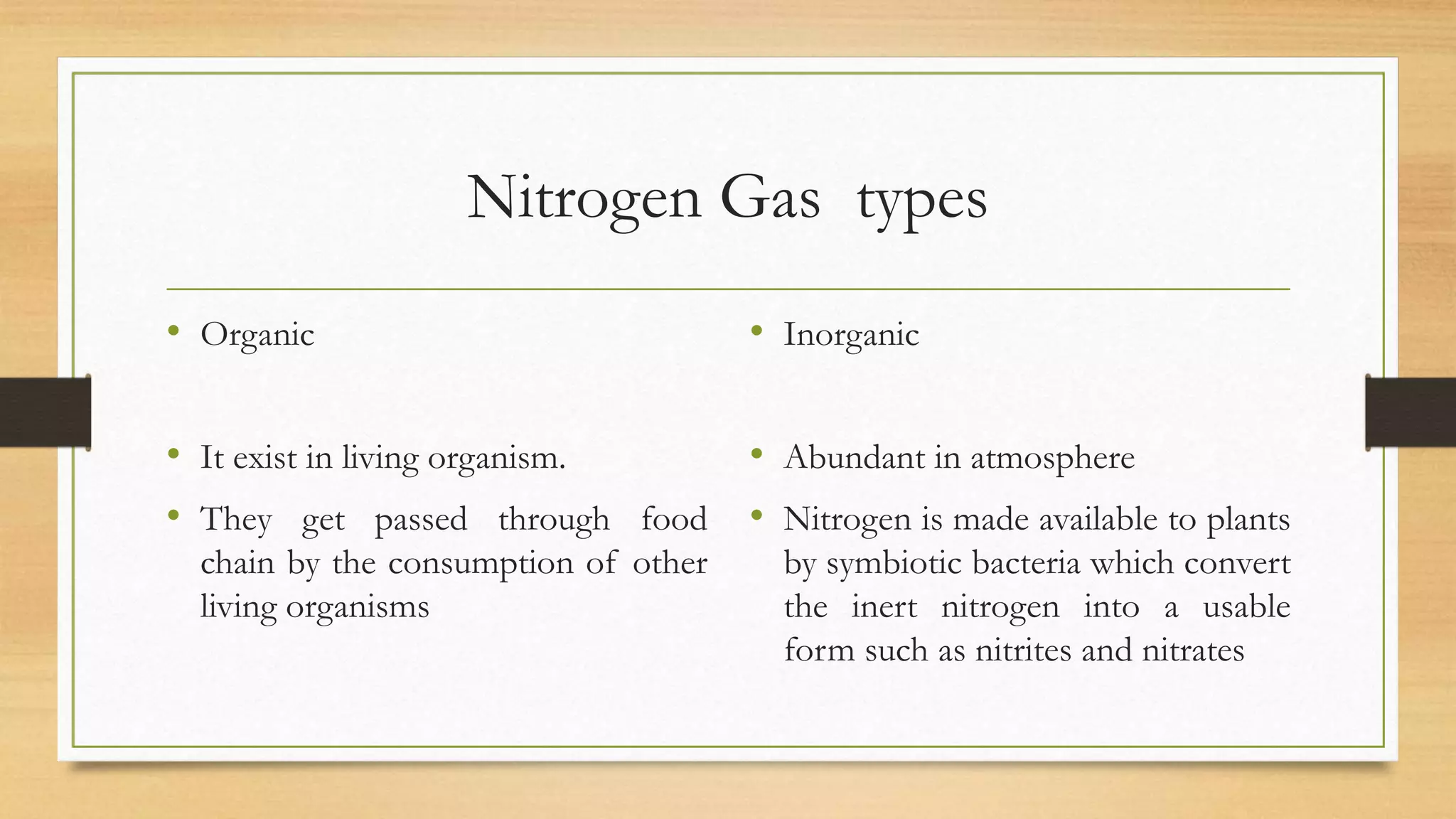
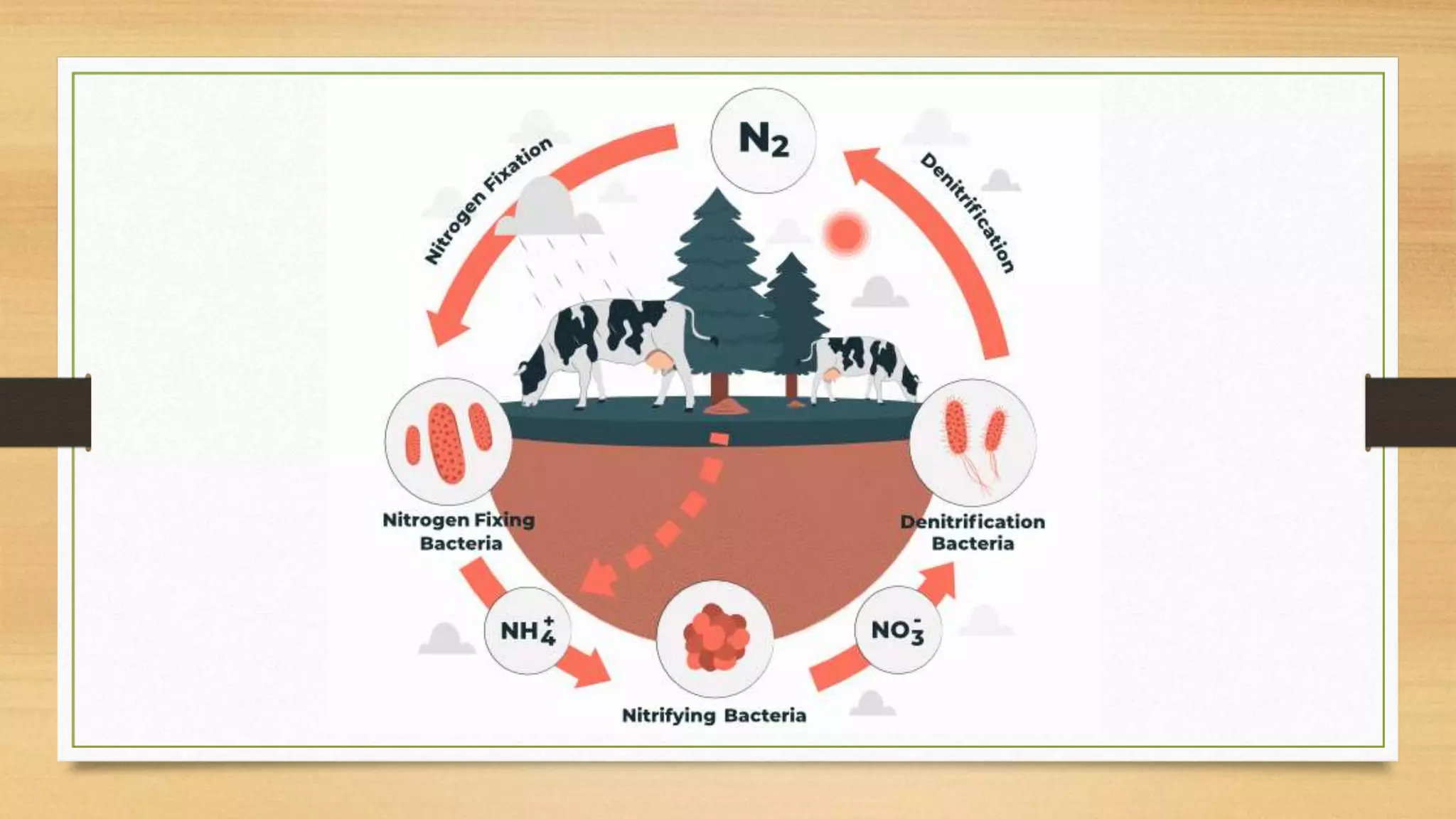
Nitrogen is a key nutrient for plants but it exists primarily as inert nitrogen gas in the atmosphere. The nitrogen cycle describes the process by which nitrogen is converted between various chemical forms as it moves between the atmosphere, soil, organisms, and various environmental reservoirs. Key steps in the nitrogen cycle include nitrogen fixation by bacteria, nitrification of ammonia to nitrites then nitrates, assimilation of nitrogen by plants and animals, ammonification of organic nitrogen waste by decomposers, and denitrification of nitrates back to nitrogen gas. The nitrogen cycle is essential for providing plants with nitrogen needed for growth while enriching soils with nutrients.