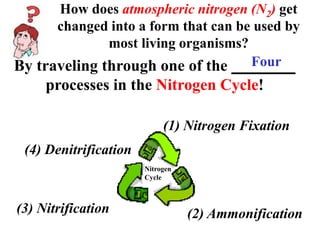
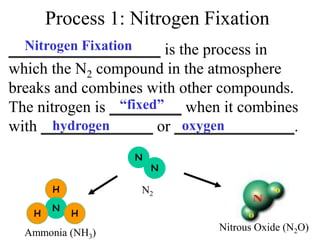
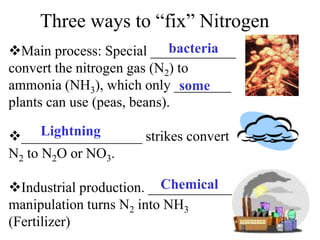
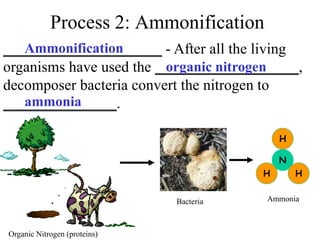
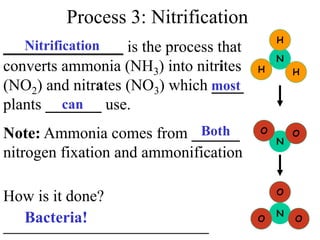
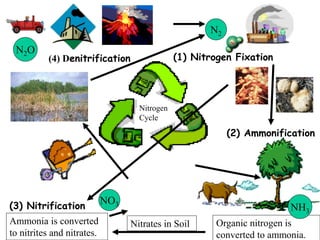
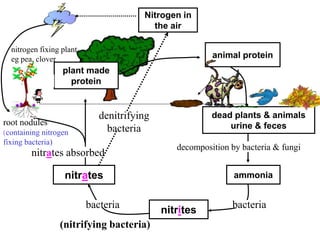
Nitrogen constitutes about 78% of the atmosphere, primarily in an unusable N2 form for plants and animals. The nitrogen cycle, comprising nitrogen fixation, ammonification, nitrification, and denitrification, enables the conversion of atmospheric nitrogen into usable compounds. Human activities, such as the use of commercial fertilizers and mining, contribute excess nitrogen, disrupting ecosystems and the food chain.