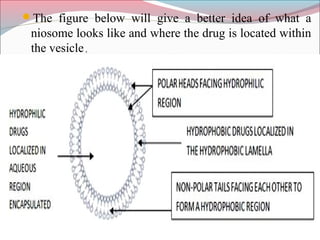
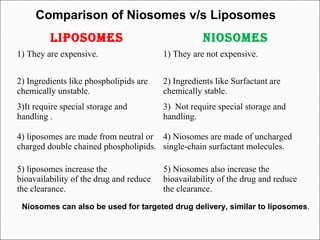
Niosomes are non-ionic surfactant vesicles that can encapsulate both hydrophilic and lipophilic drugs. They are similar to liposomes but are more stable and less expensive. Niosomes are microscopic lamellar structures composed of non-ionic surfactants and cholesterol. They can be unilamellar or multilamellar depending on the preparation method. Niosomes increase the bioavailability and delivery of drugs while reducing side effects and can be used for targeted drug delivery applications like cancer treatment.