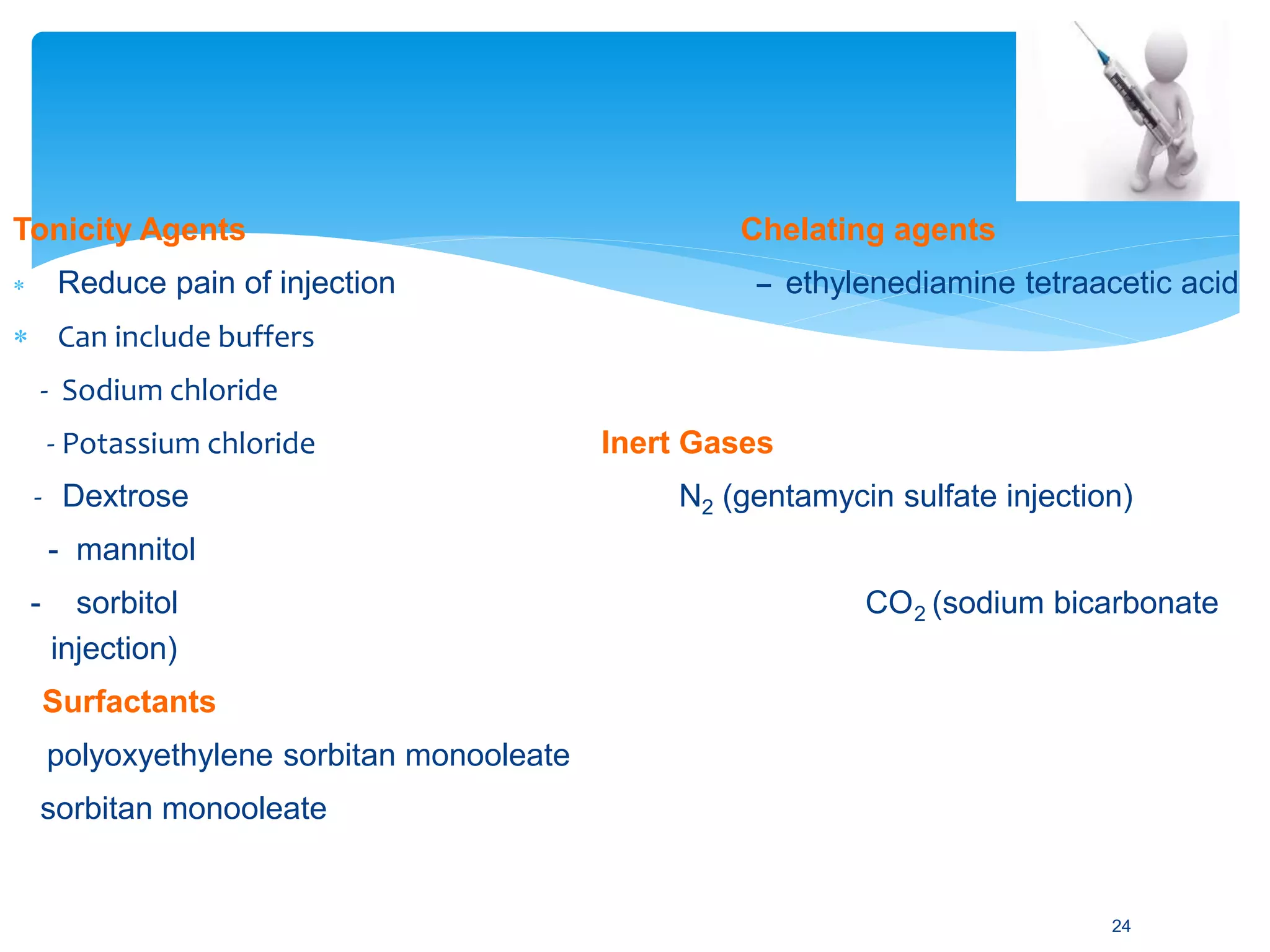
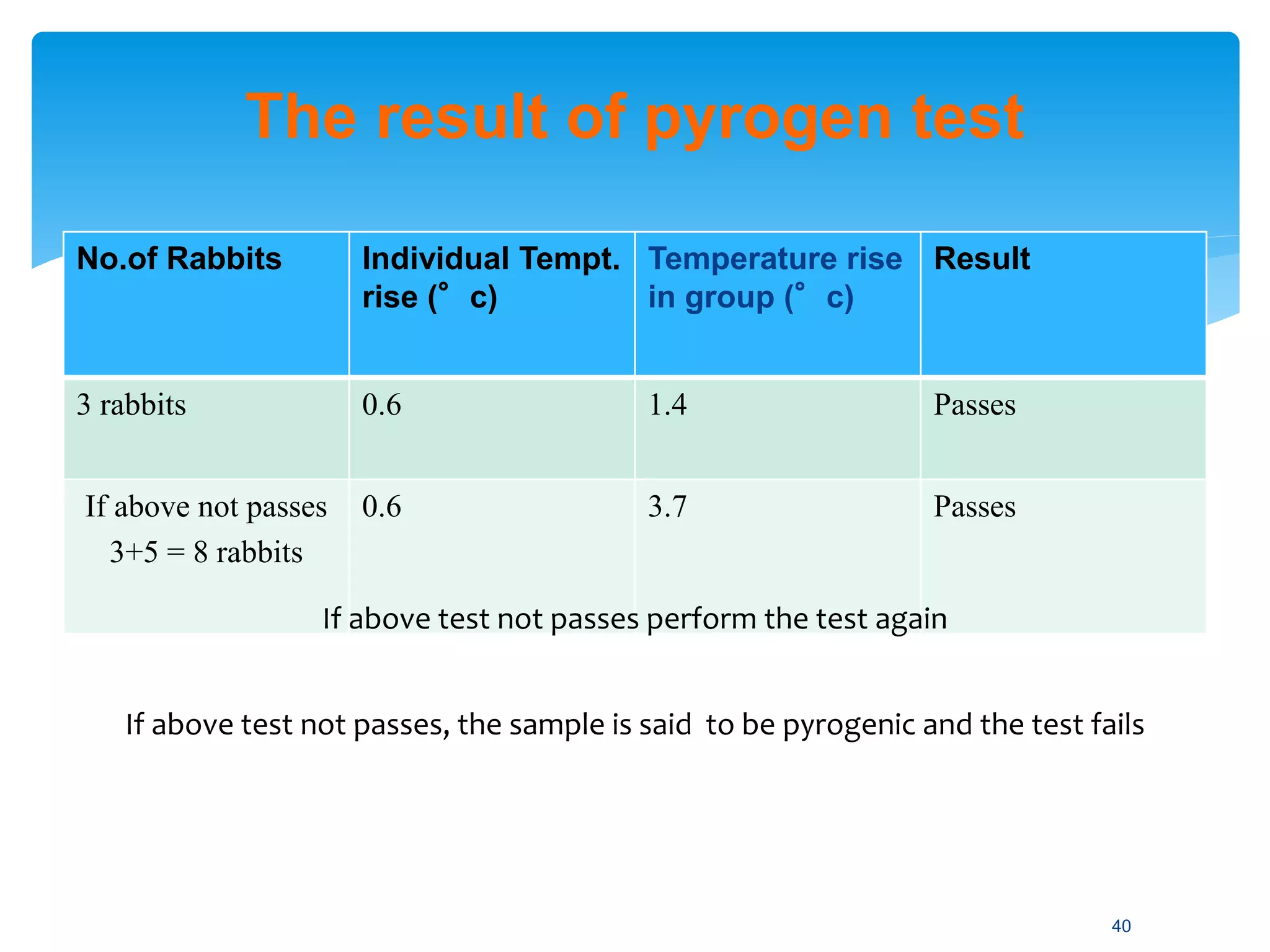
The document discusses parenteral preparations, highlighting their definitions, routes of administration, advantages, disadvantages, formulation, manufacturing processes, and quality control measures. Parenterals are injectable, sterile preparations that bypass the gastrointestinal tract, and can be administered through various routes such as subcutaneous, intramuscular, and intravenous. It emphasizes the importance of sterility, isotonicity, and quality testing directly affecting patient safety and treatment efficacy.