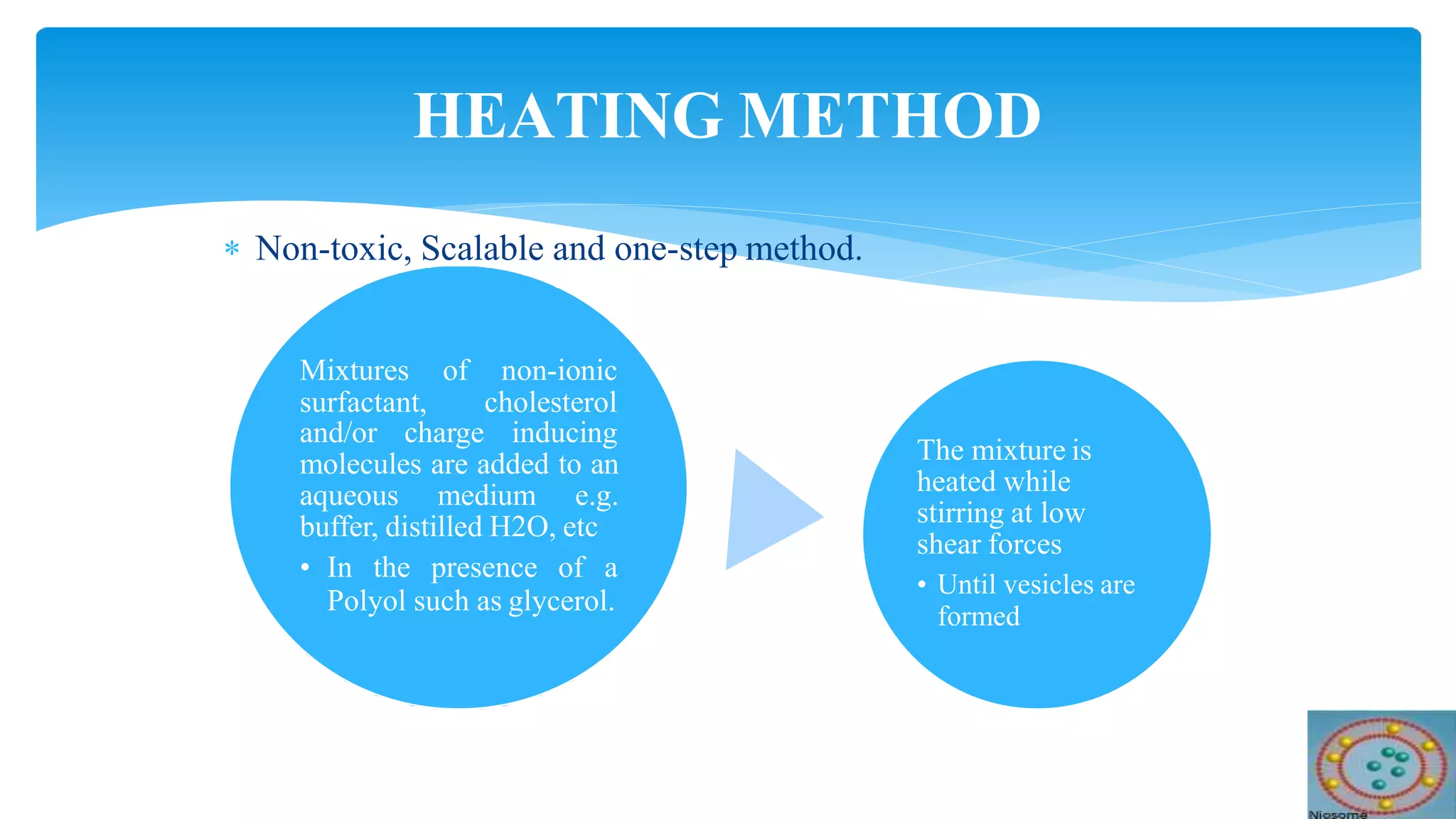
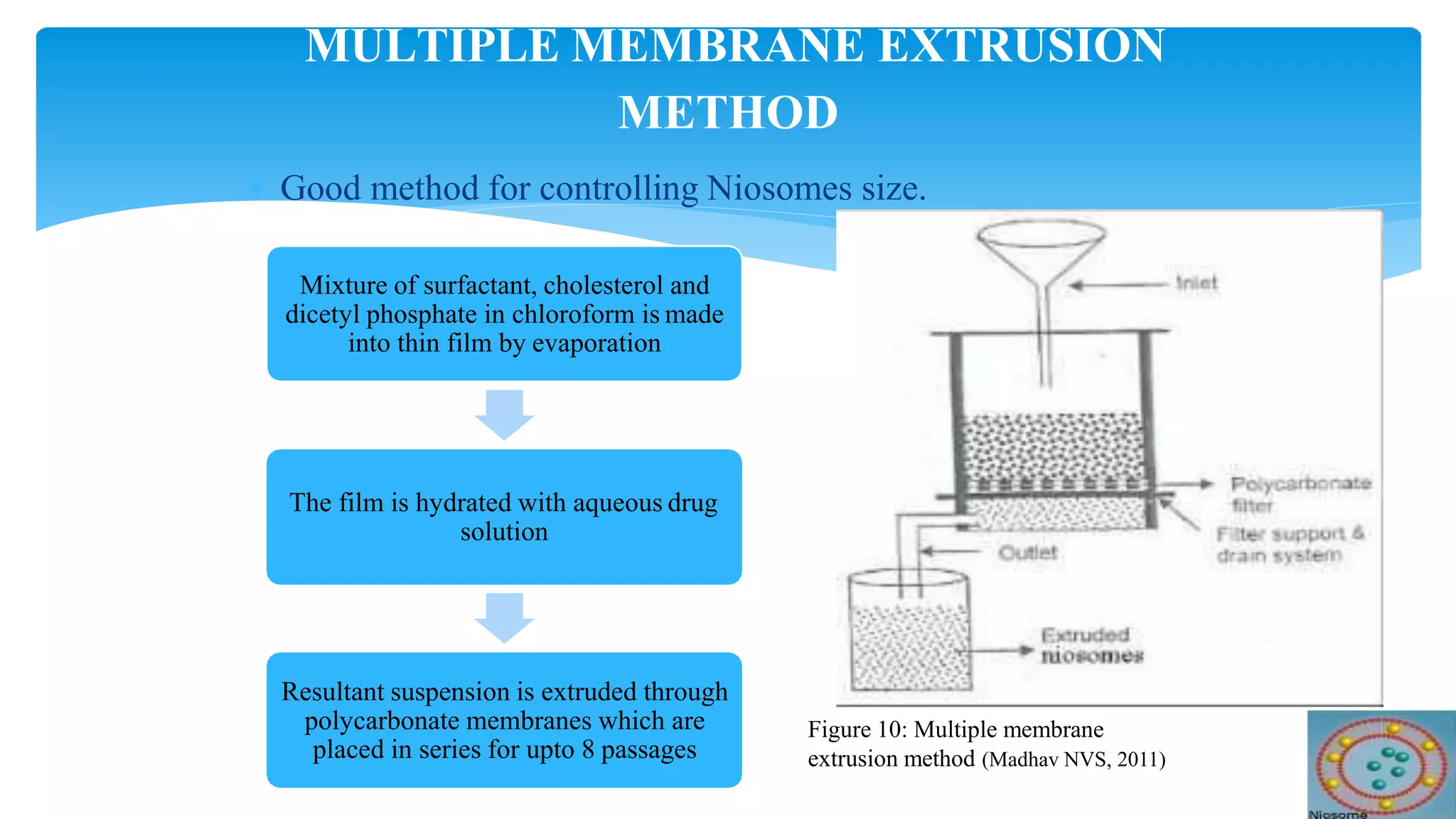
Niosomes are novel drug delivery vesicles made of non-ionic surfactants that can encapsulate both hydrophilic and hydrophobic drugs. They have a similar bilayer structure to liposomes but are more stable and less expensive. Niosomes can improve the therapeutic performance of drugs by protecting drugs from degradation and restricting effects to target cells. Various preparation methods are used to produce niosomes of different sizes and lamellarity that can be used to deliver drugs through different routes of administration like oral, parenteral, topical to treat diseases like cancer, infections and more.