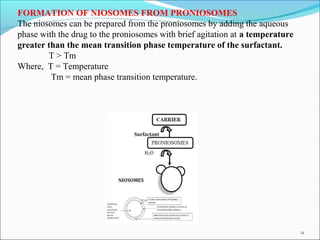
Niosomes are synthetic microscopic vesicles made from nonionic surfactants and cholesterol, used as drug delivery systems due to their stability and lower toxicity compared to liposomes. They can accommodate a wide range of drug solubilities and improve therapeutic performance by controlling drug release and target delivery. The document discusses their structure, preparation methods, advantages, and applications in various medical treatments.