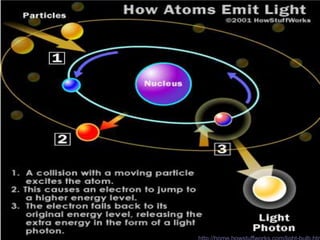
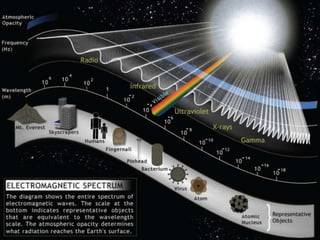
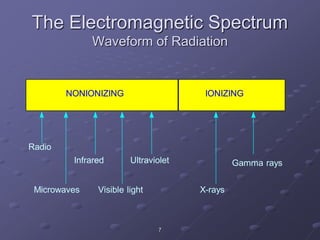
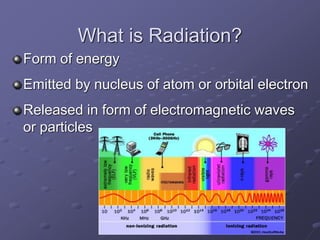
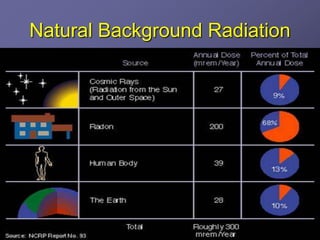
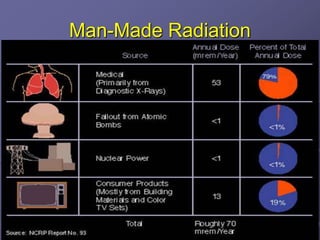
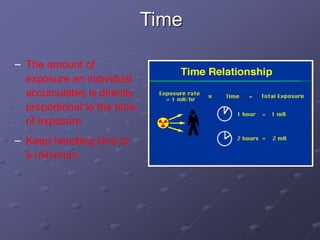
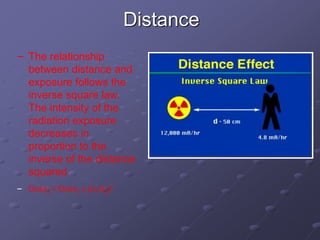
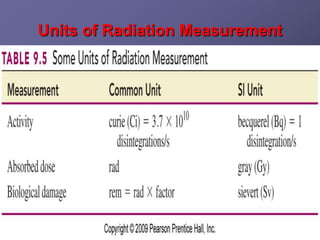
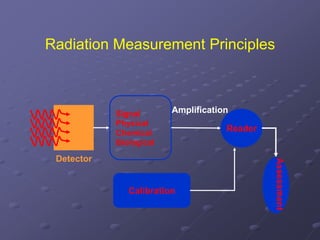
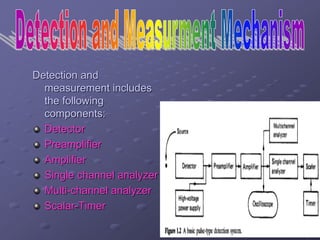
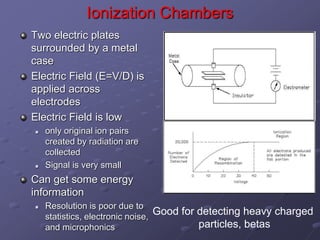
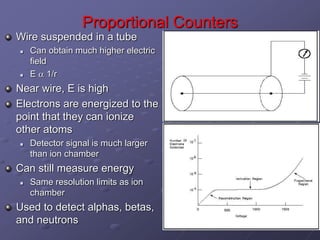
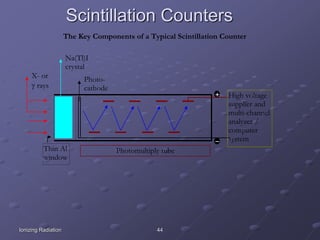
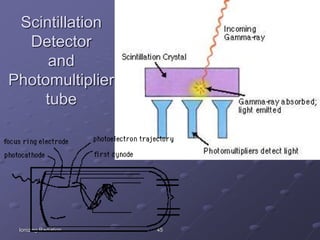
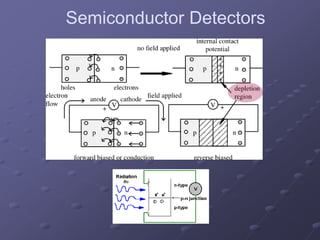
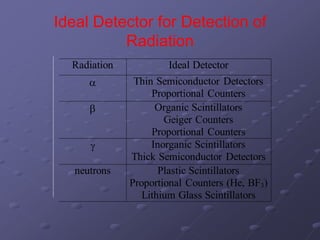
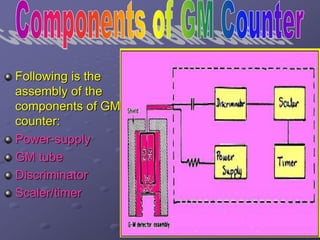
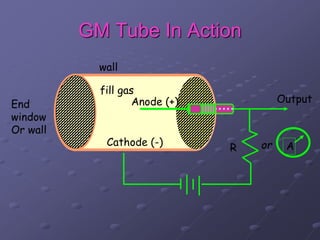
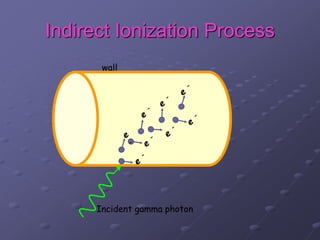
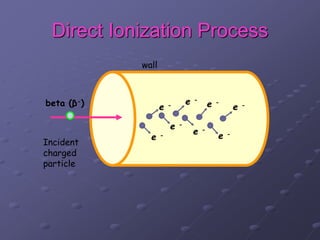
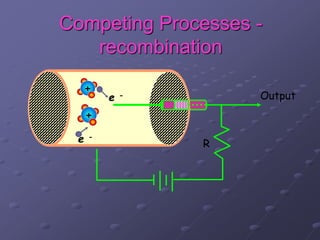
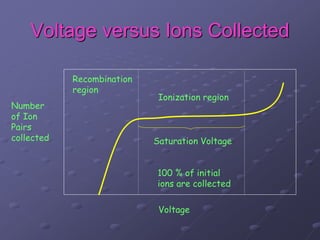
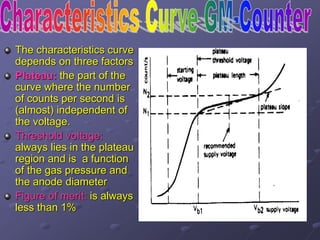
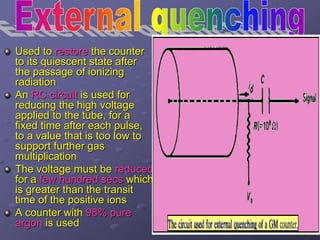
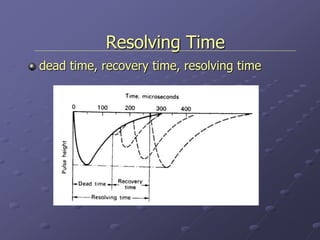
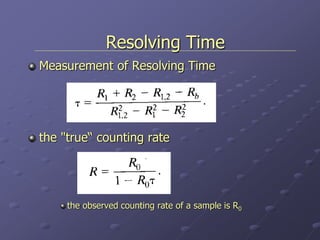
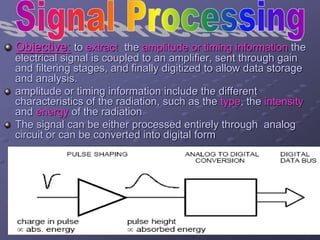
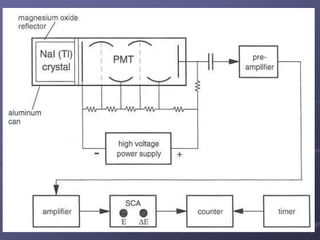
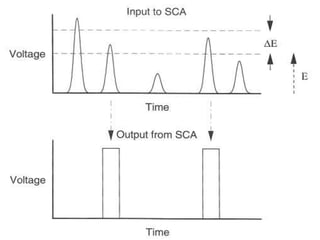
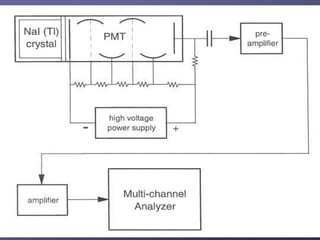
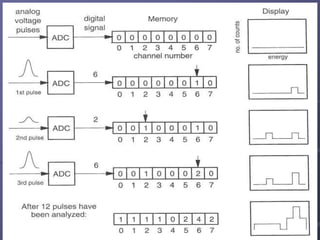
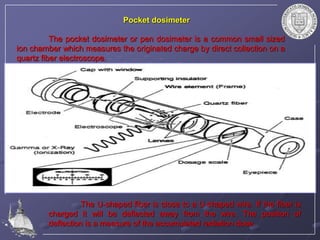
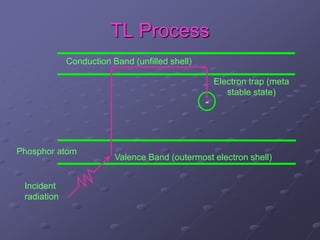
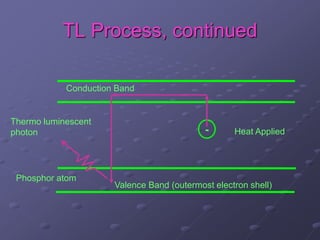
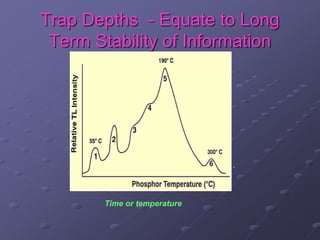
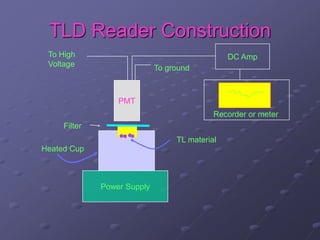
Radiation detectors work by exploiting how radiation interacts with matter to produce measurable signals. The document discusses several types of radiation detectors, including gas-filled detectors like Geiger-Muller counters, scintillation detectors, and semiconductor detectors. It explains how each detector works and its applications, advantages, and limitations. The document also covers topics like pulse processing, resolving time, and quenching in Geiger counters to restore the detector to a quiescent state between detections.