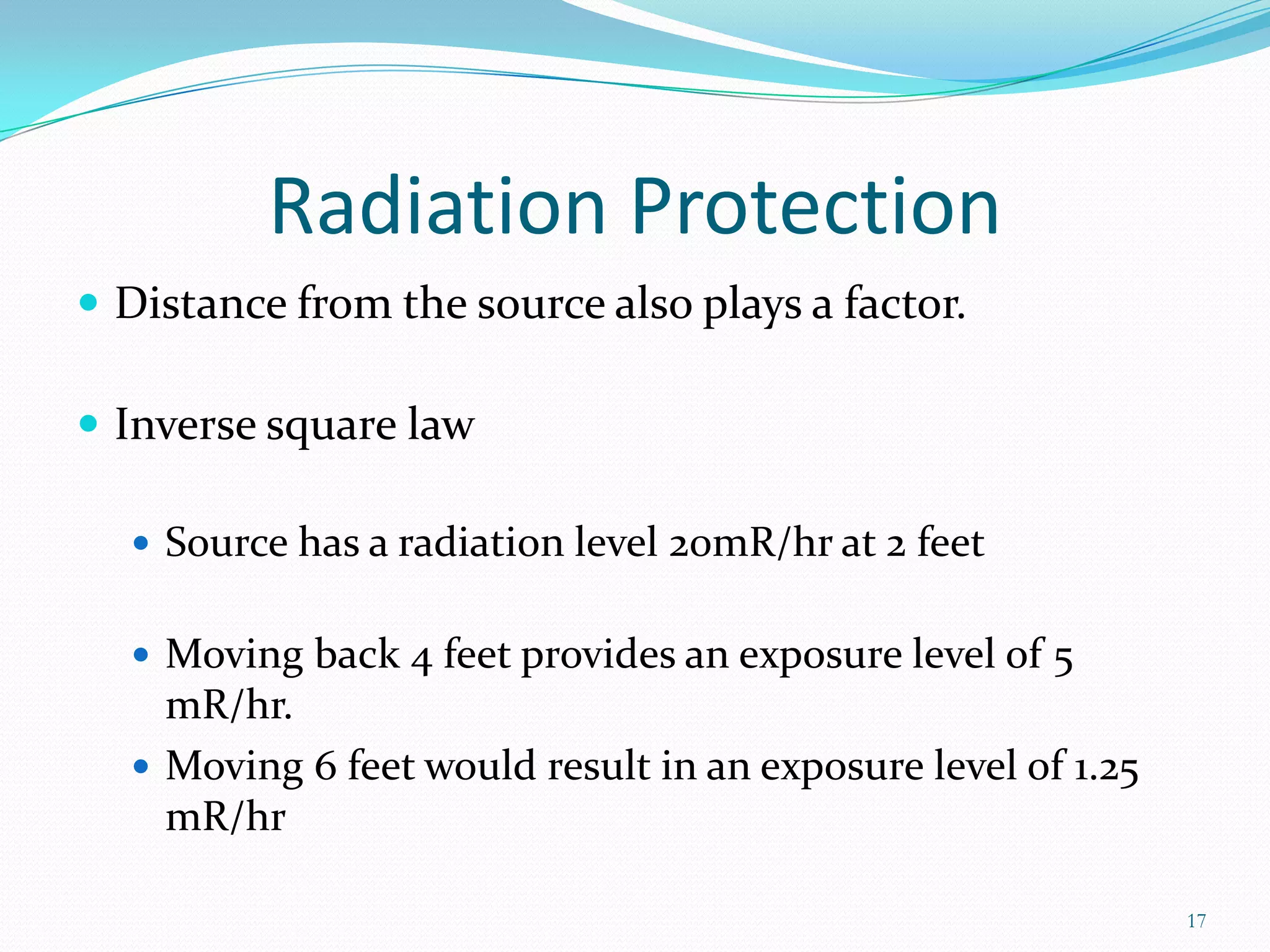
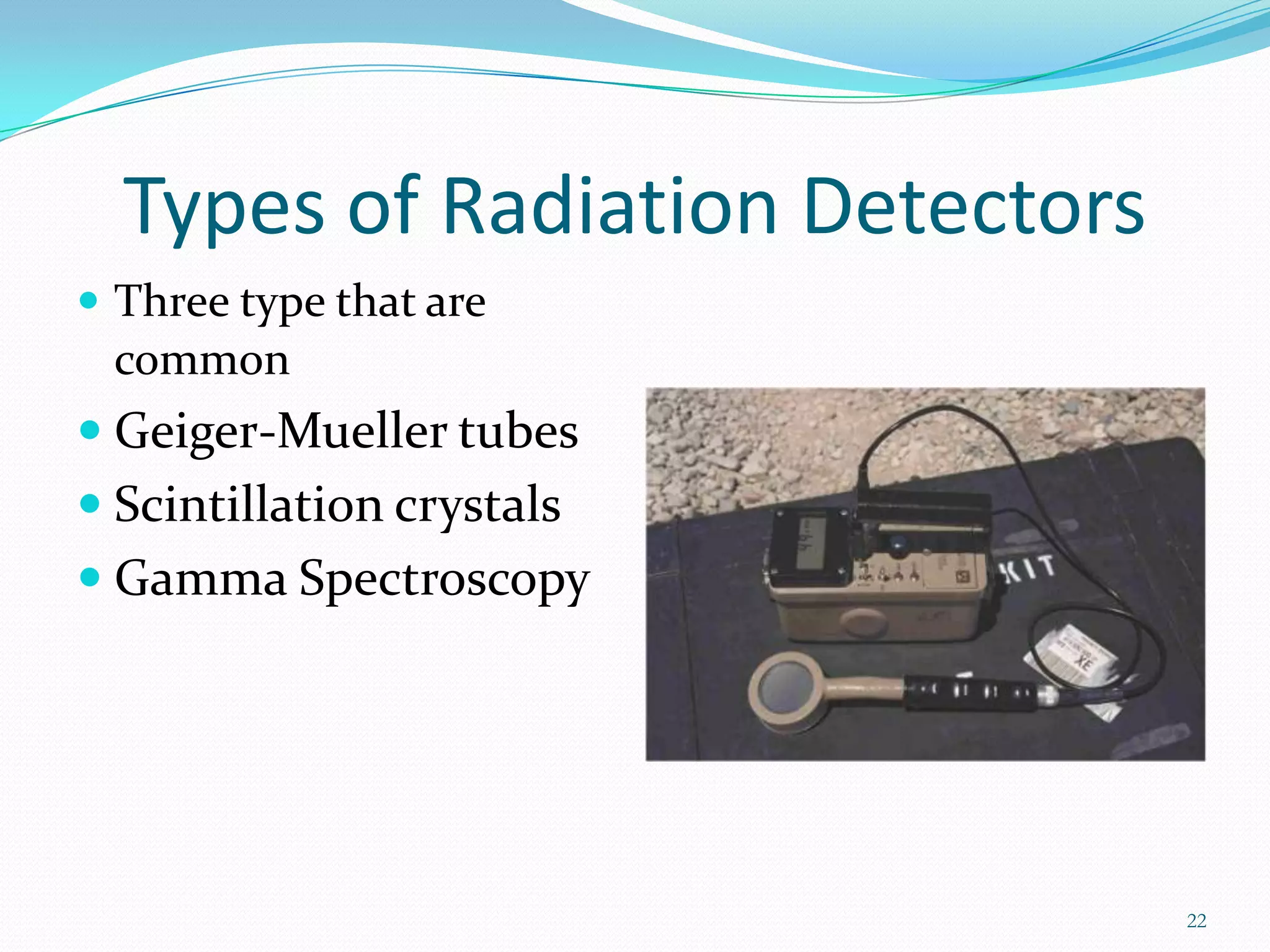
This document discusses radiation sources, types of radiation, and radiation detection devices for emergency responders. It identifies common radiation sources, describes how radiation can impact humans, and defines key radiation measurement terms like absorbed dose, equivalent dose, and half-life. The document outlines different types of radiation detectors including Geiger-Mueller tubes, scintillation crystals, and gamma spectroscopy devices. It stresses that responders need training to understand radiation monitoring and detection to safely respond to potential radiation incidents.