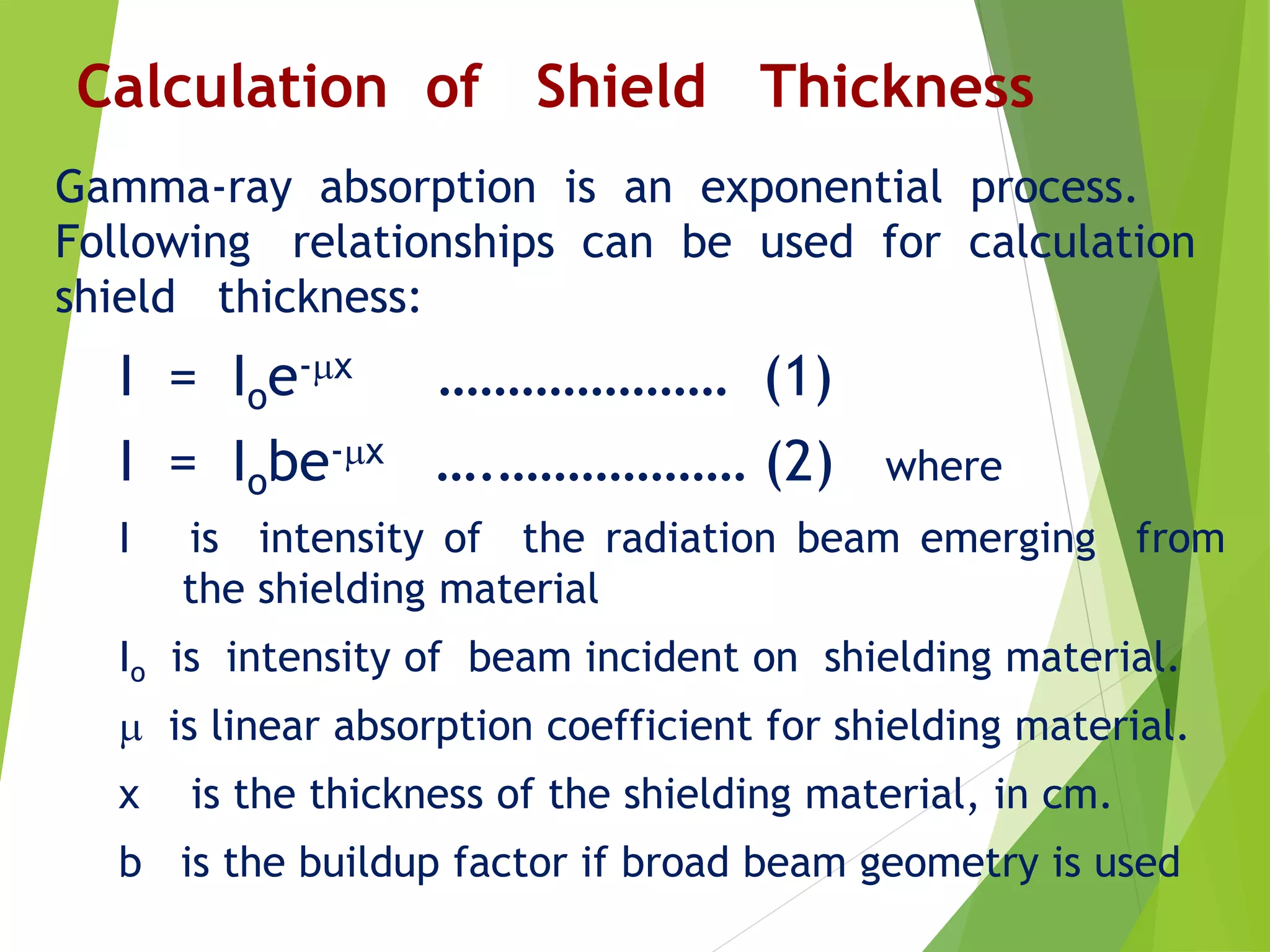
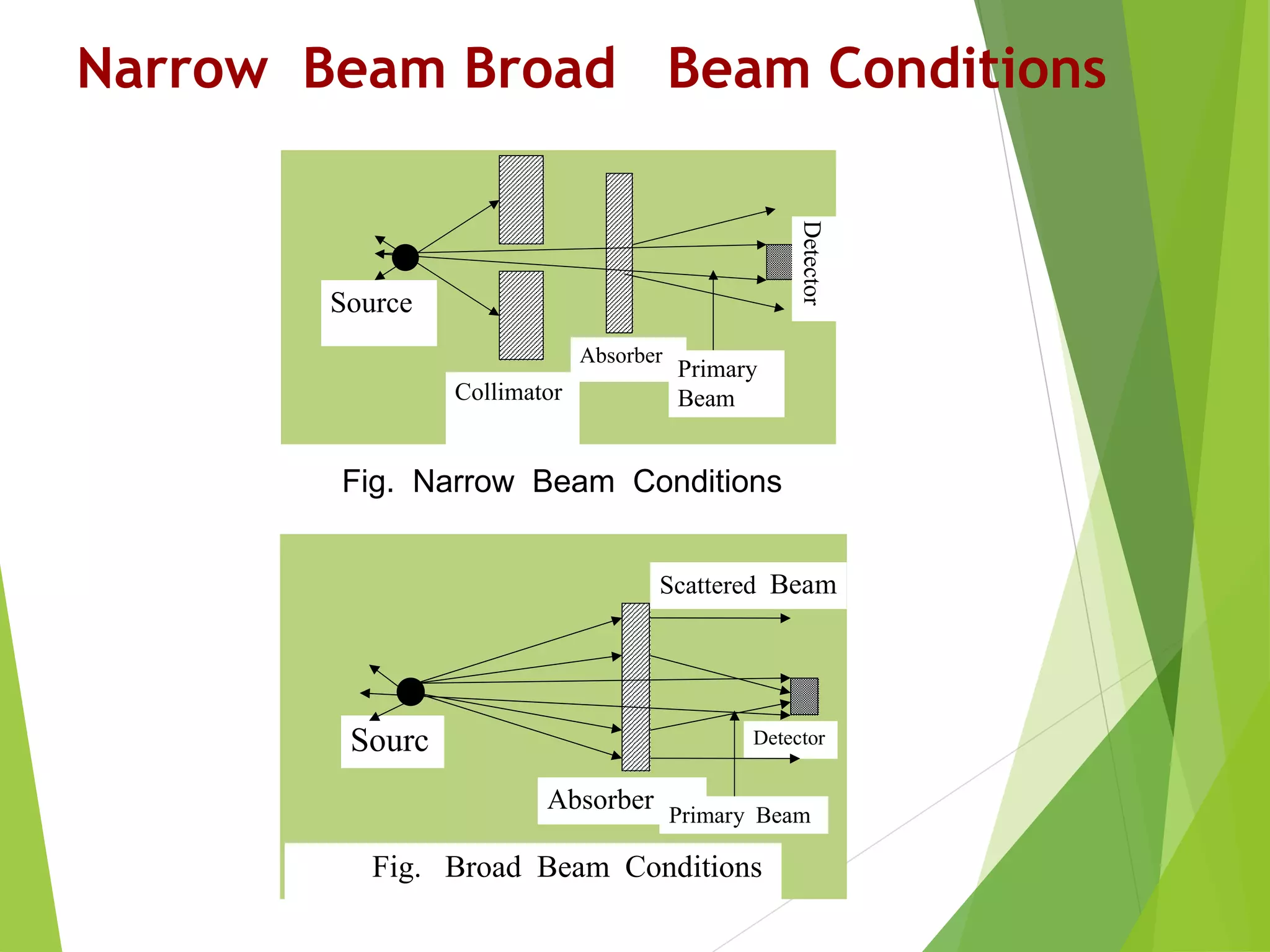
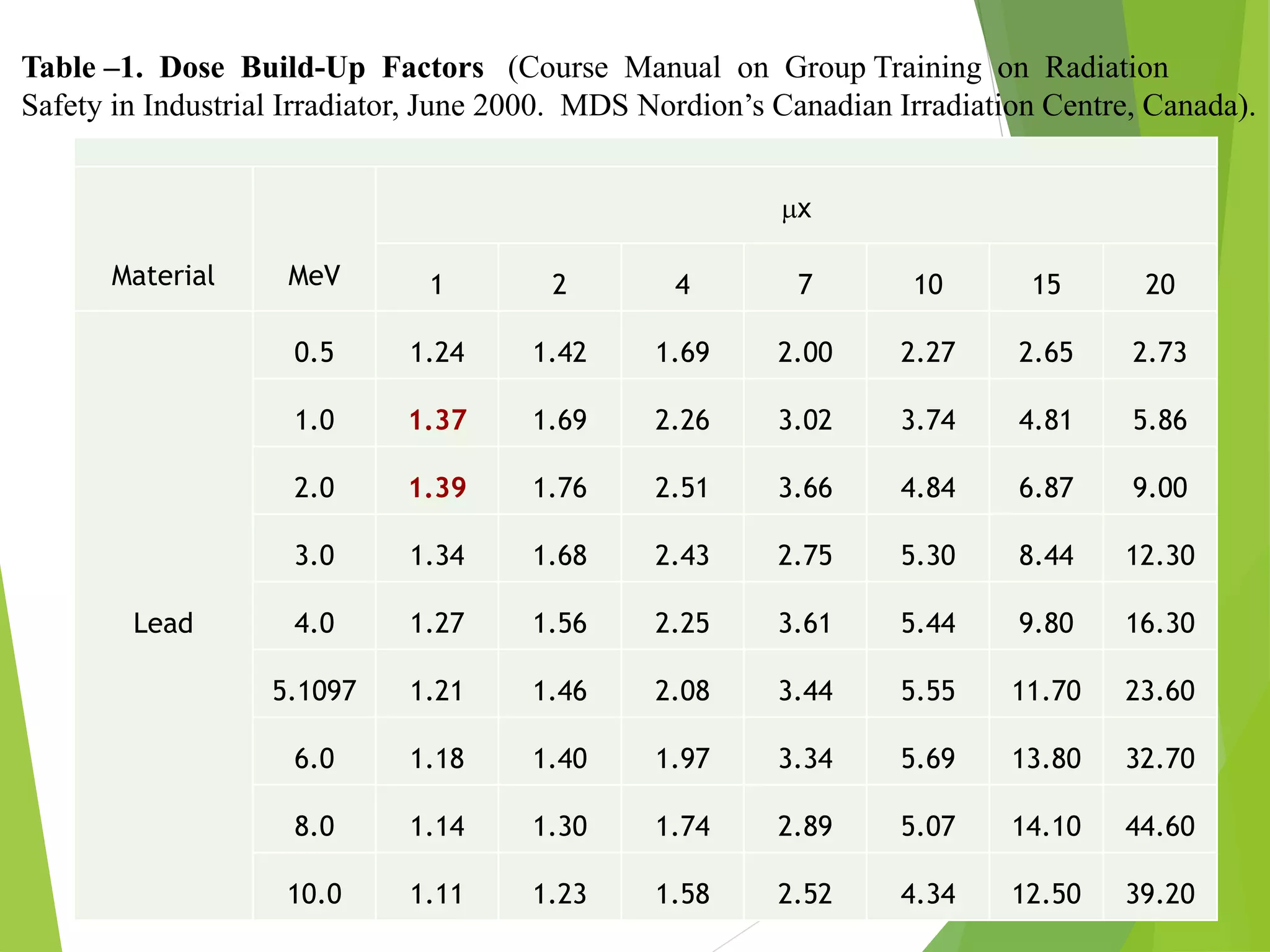
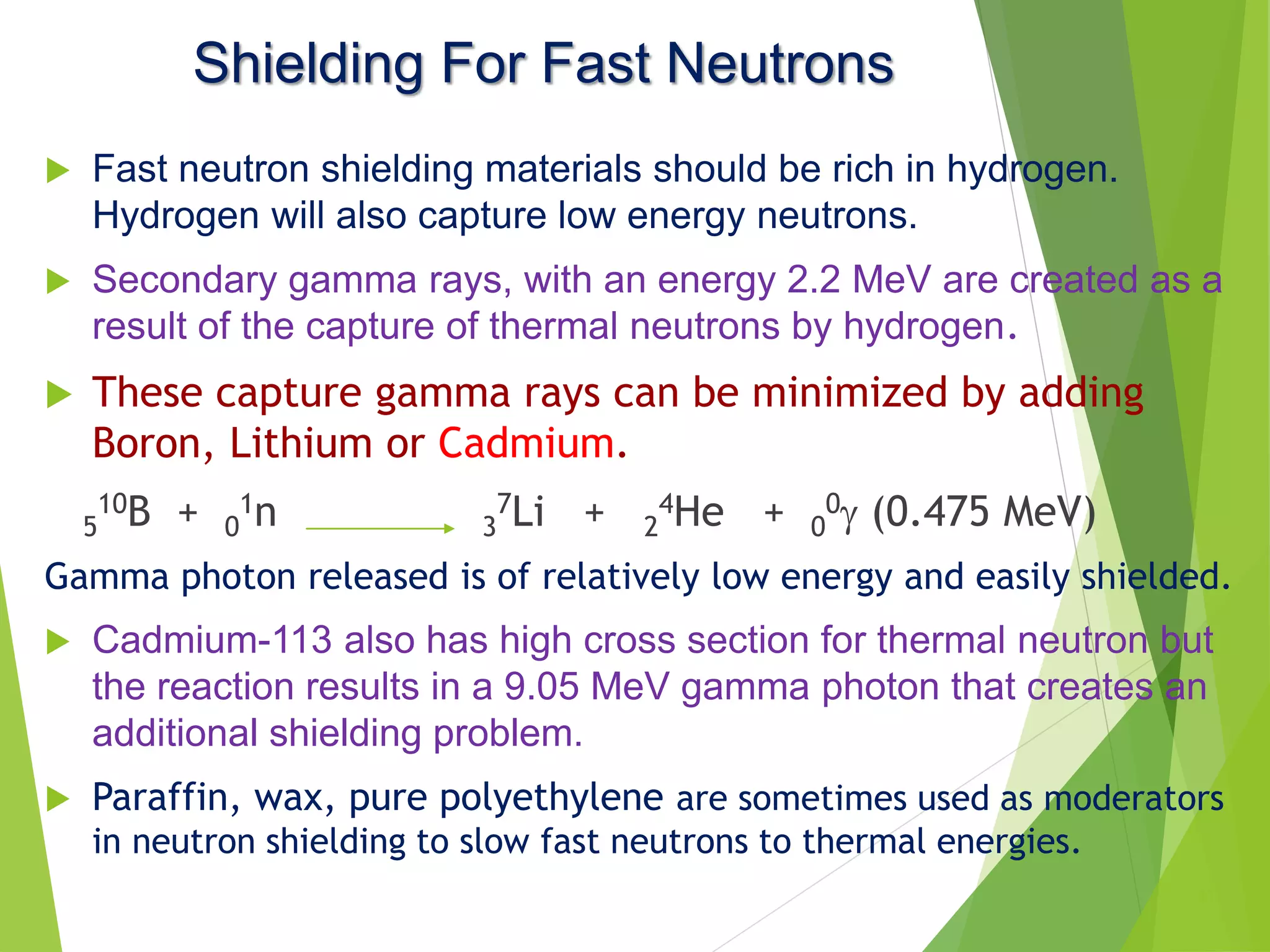
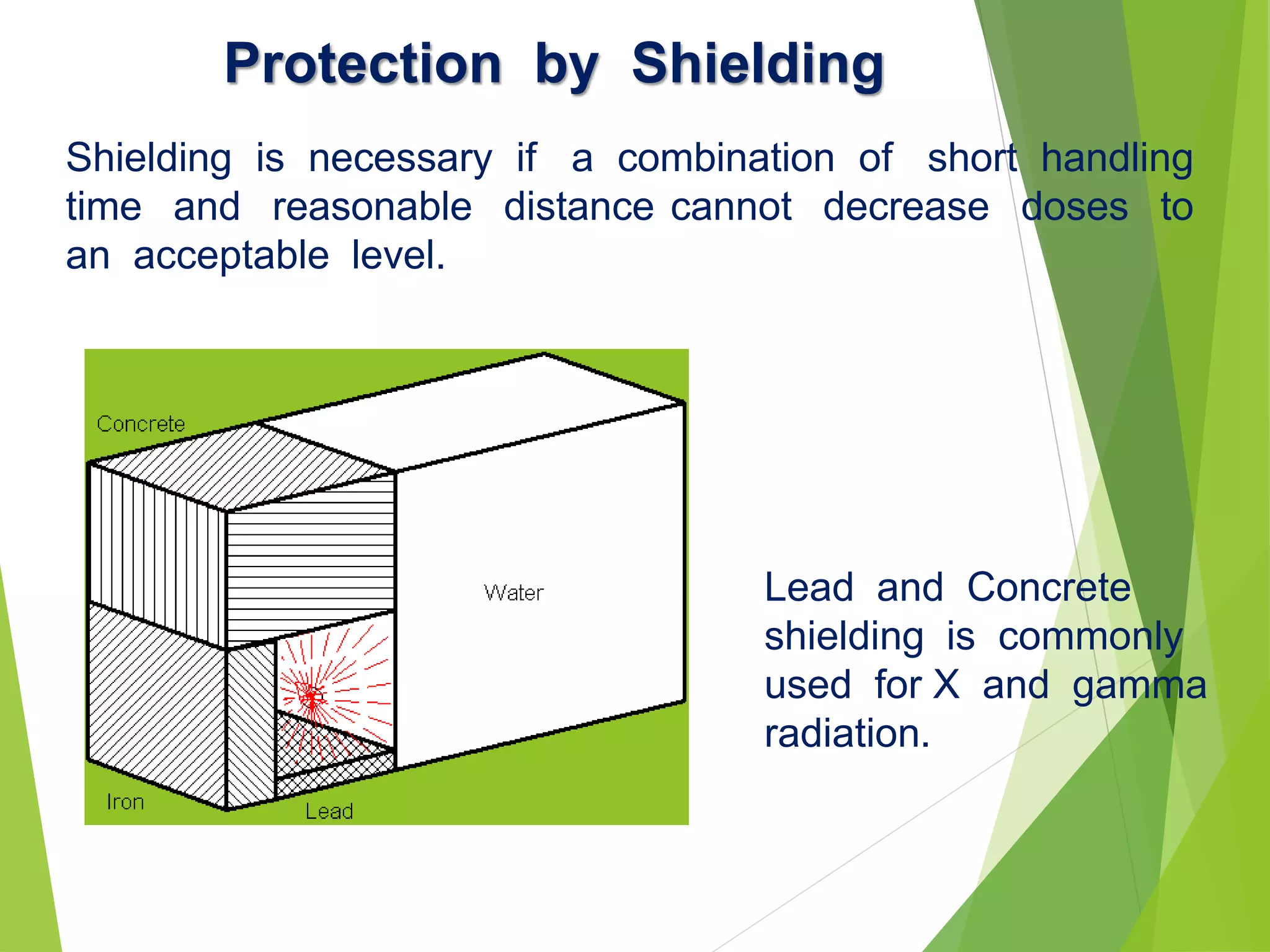
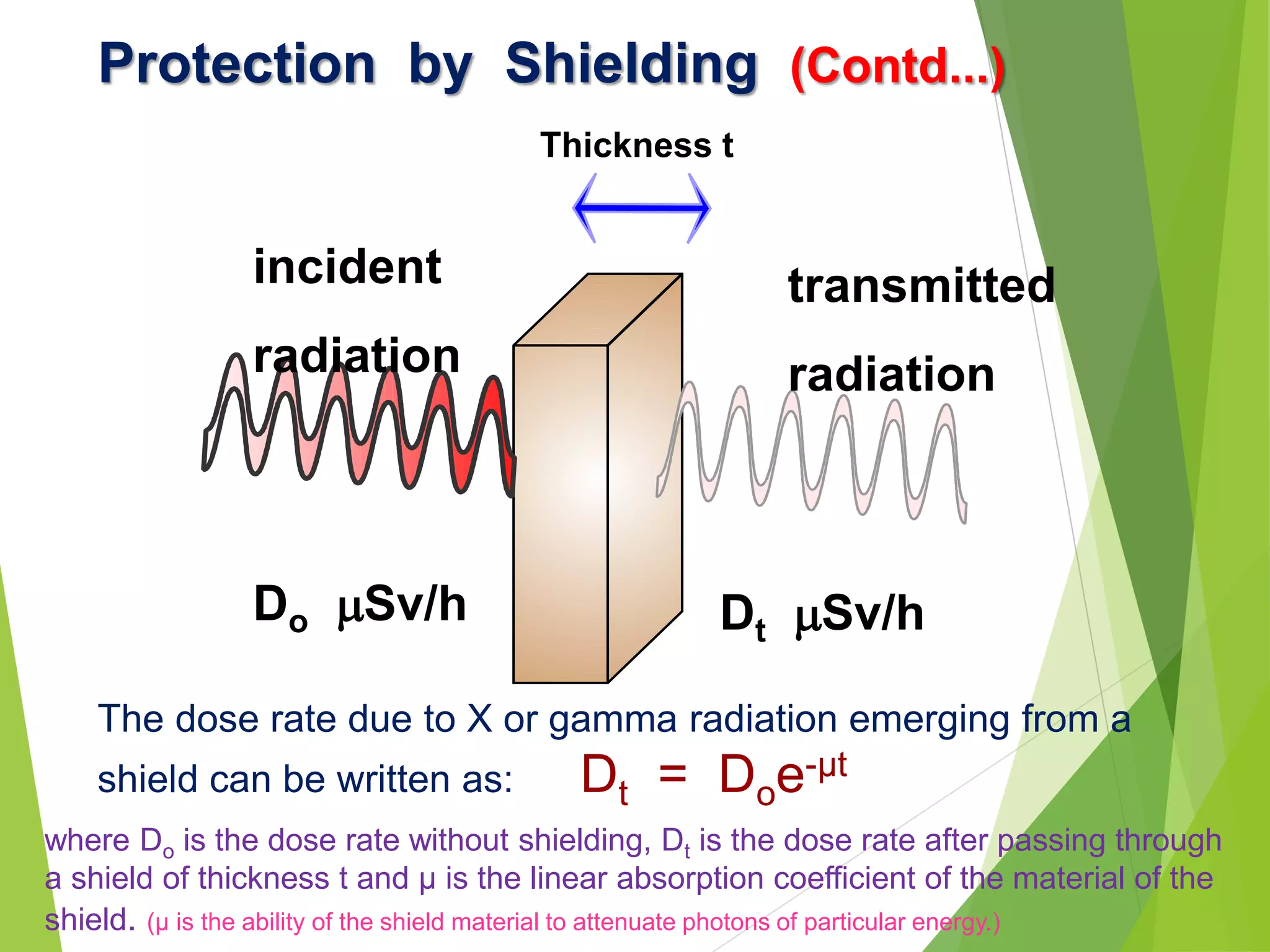
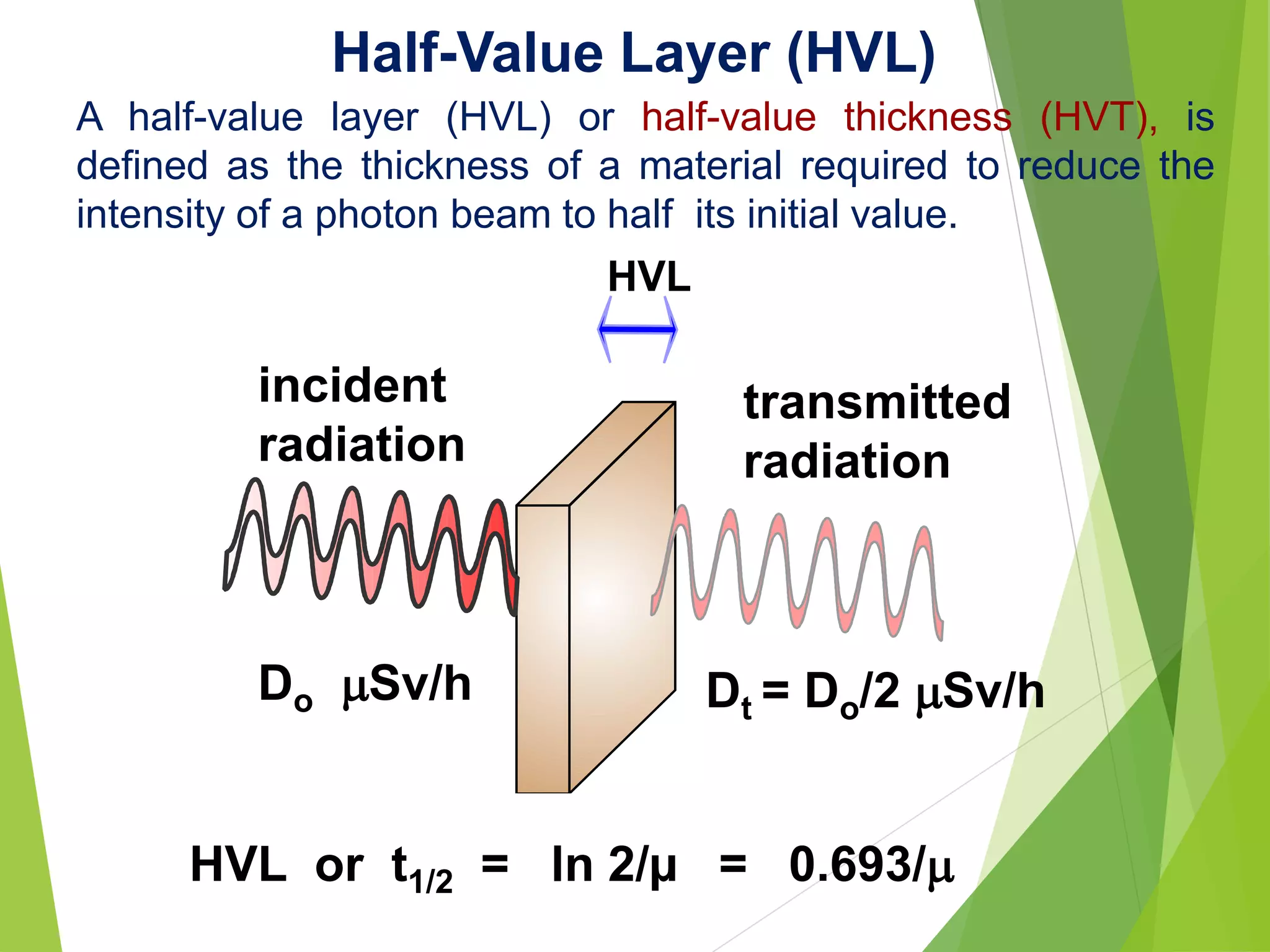
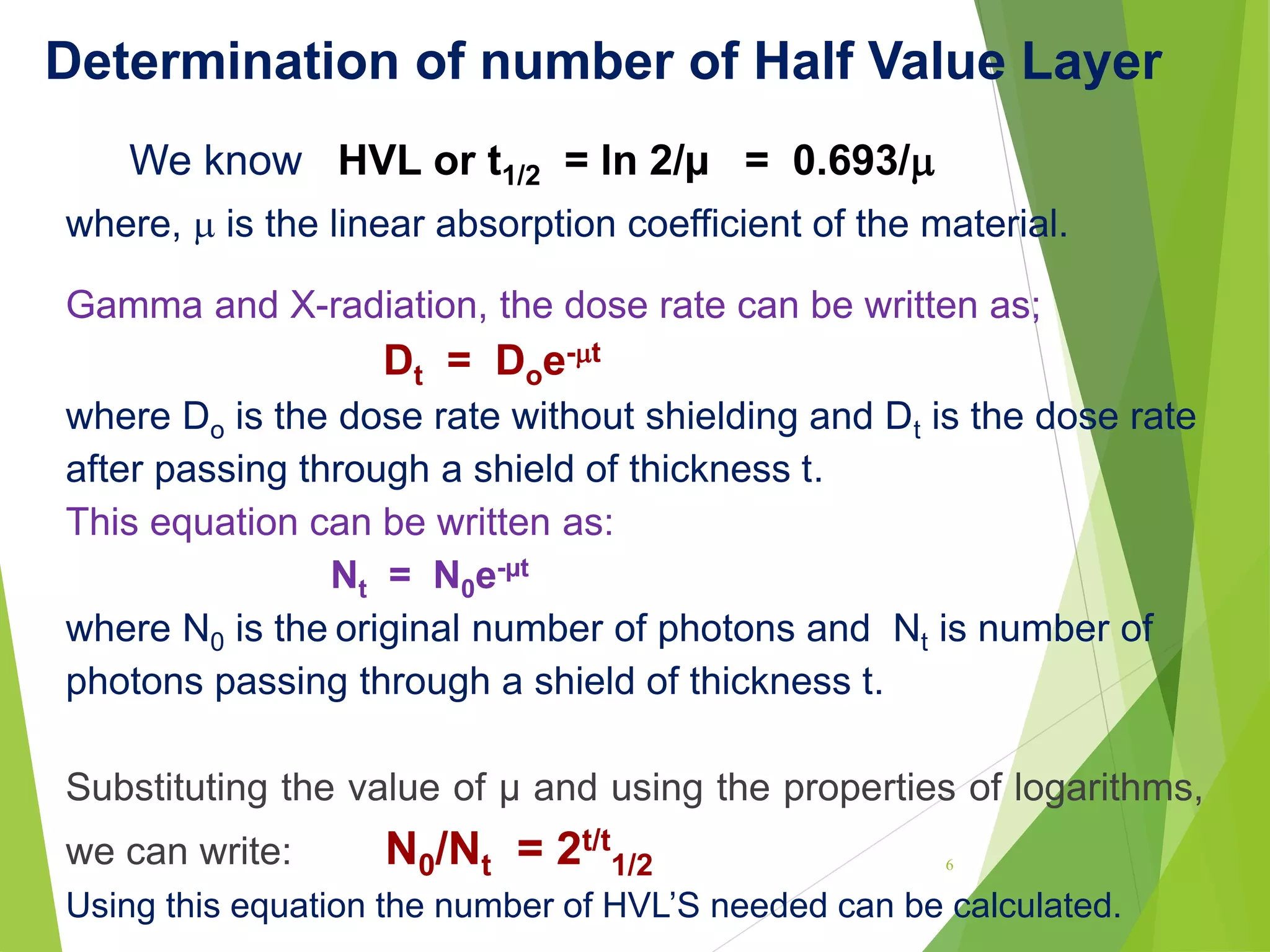
This document discusses radiation shielding concepts and calculation techniques necessary for protecting against radiation hazards, particularly for x and gamma radiation. It covers the importance of shielding materials, calculations for determining the thickness of shields required, and provides examples and problems related to radiation dose reduction through shielding. Additionally, it addresses half-life concepts and fast neutron shielding methods, emphasizing the need for materials rich in hydrogen for effective protection.


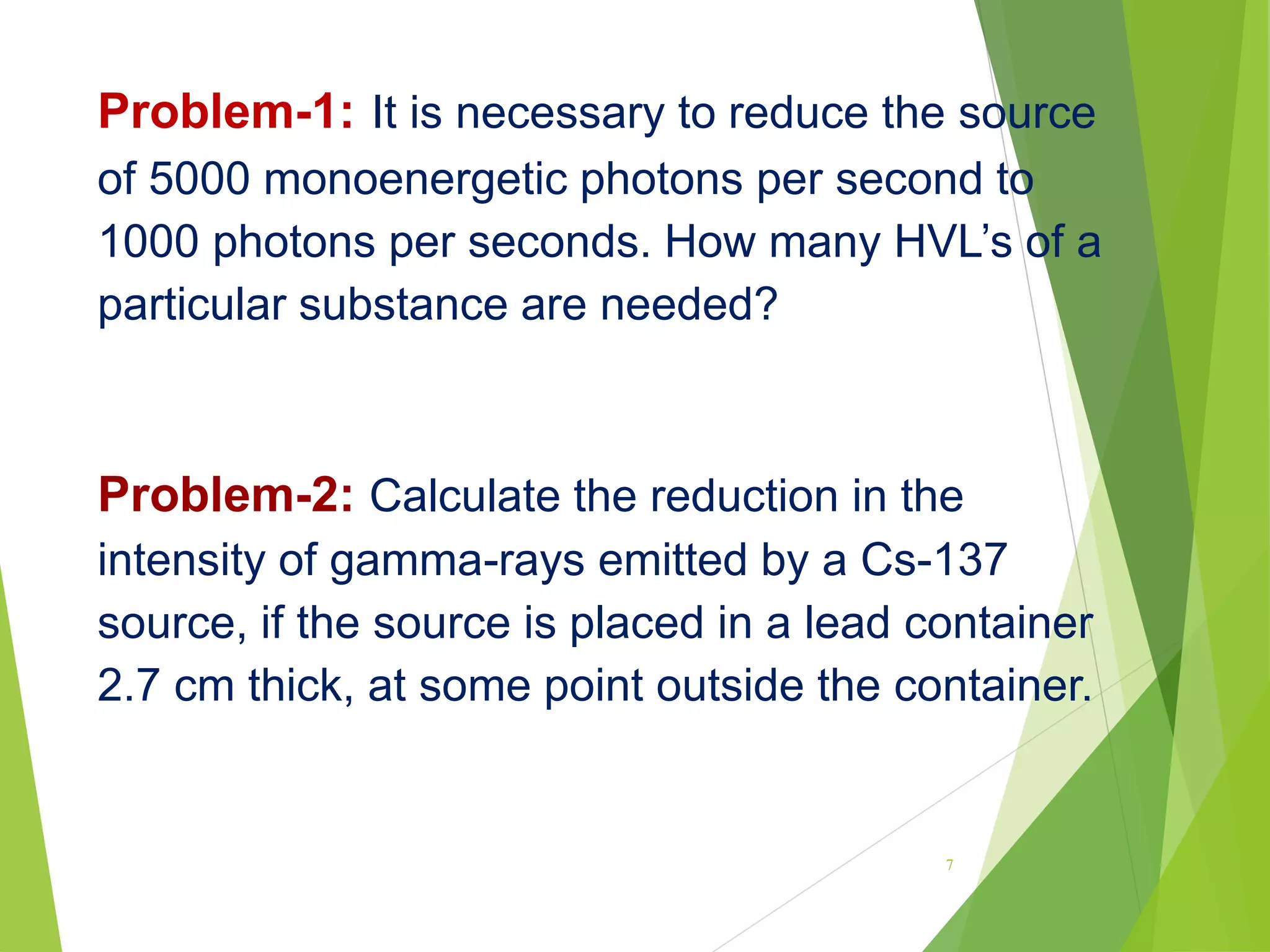

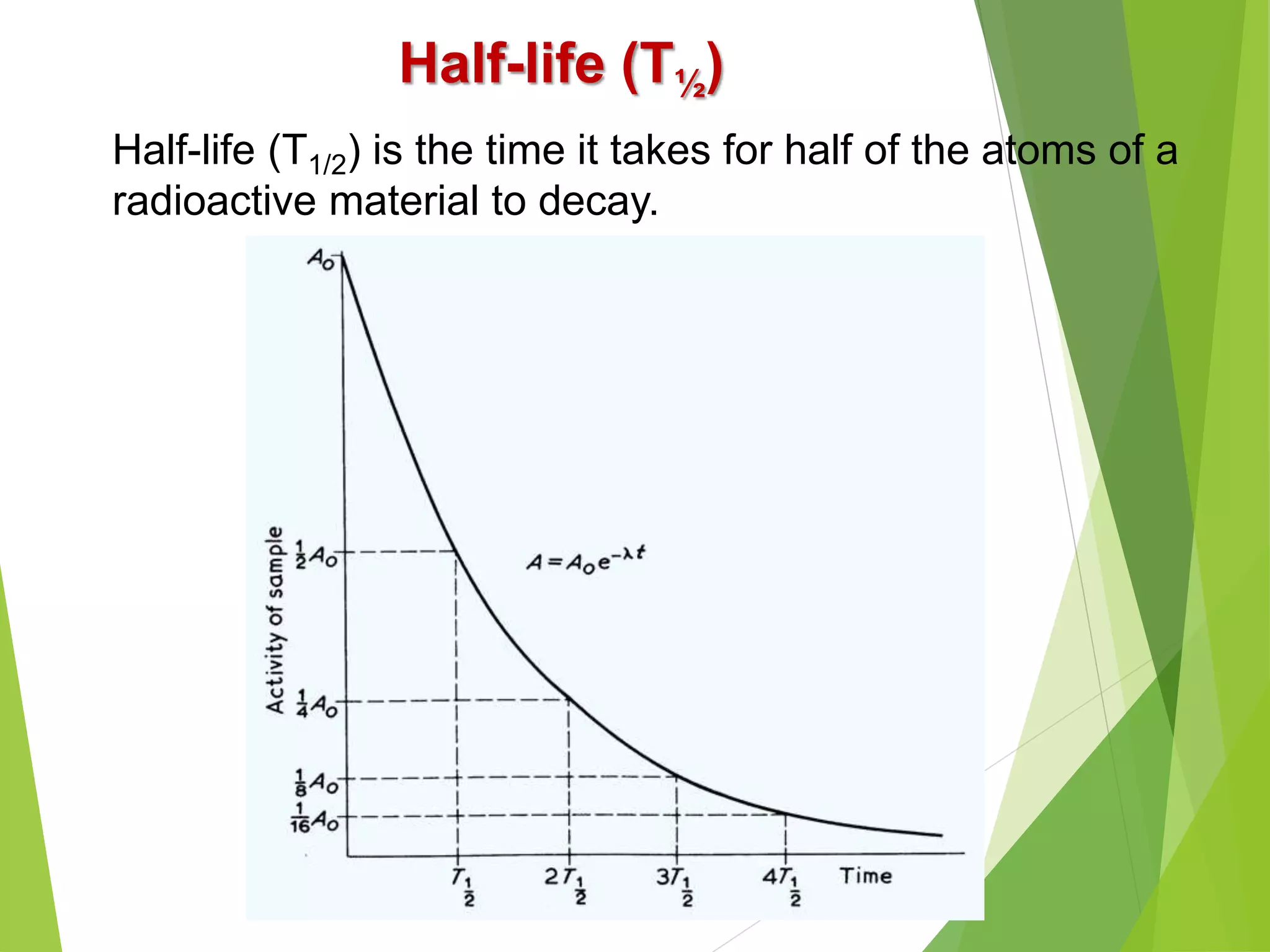
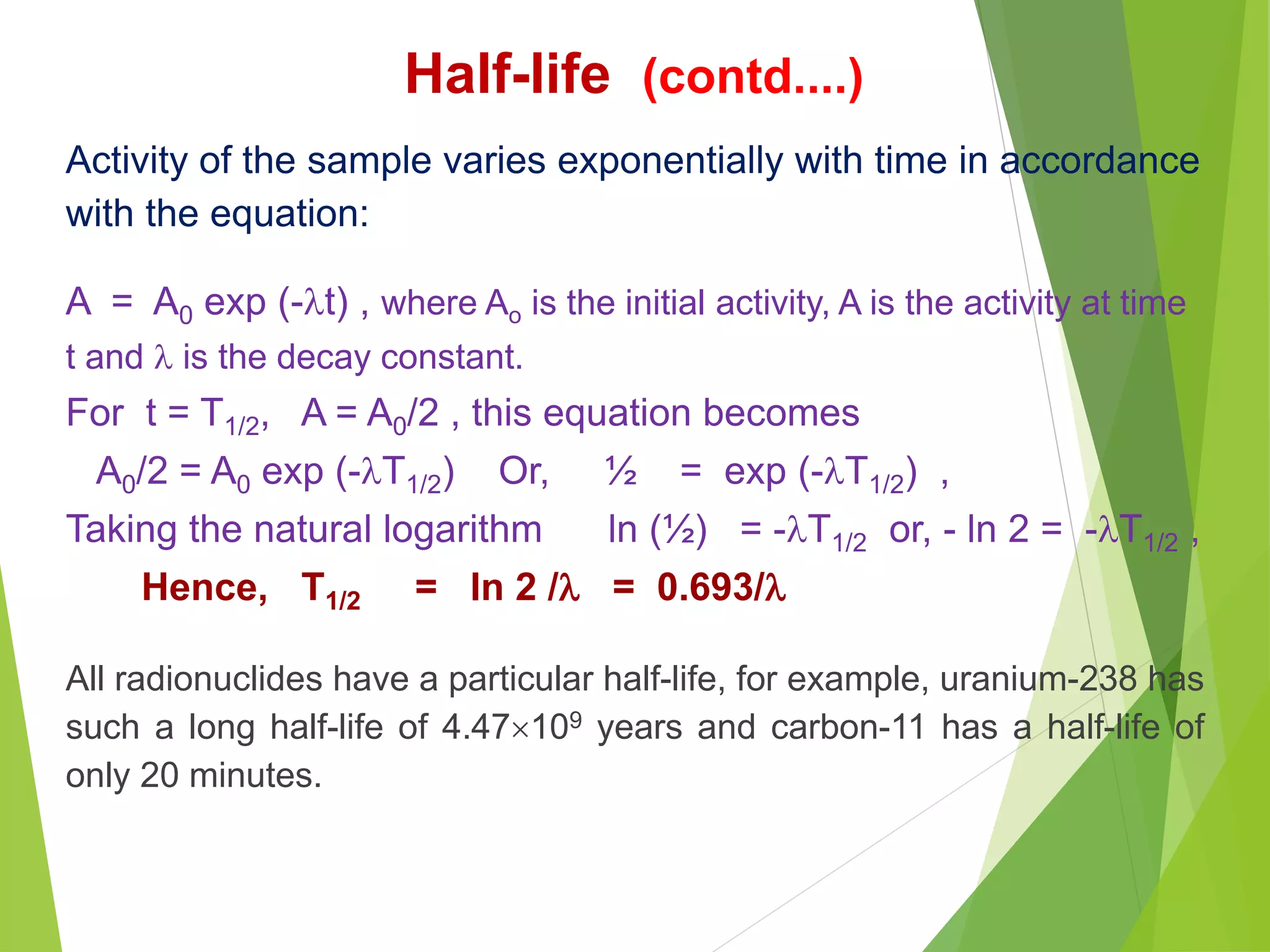
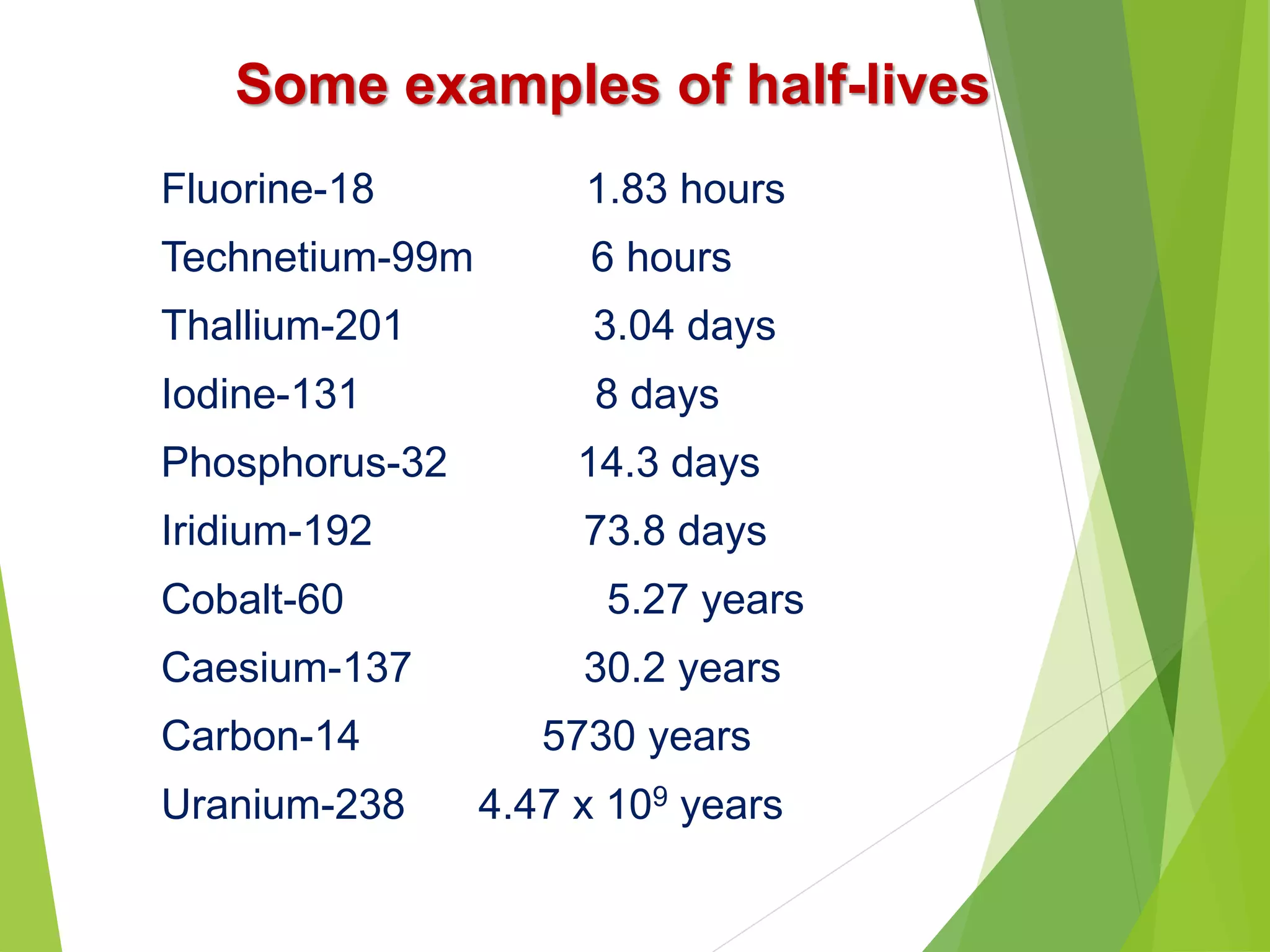
![Problem-4: Calculate the activity of 30 mCi source of
11
24Na after 2.5 days. What is the decay constant of this
radionuclide? [ Half life = 15 hr]
12](https://image.slidesharecdn.com/basicconceptofradiationshielding-211104165010/75/BASIC-CONCEPT-OF-RADIATION-SHIELDING-AND-ITS-CALCULATION-TECHNIQUES-12-2048.jpg)