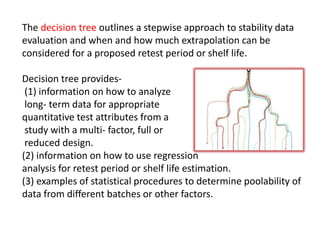
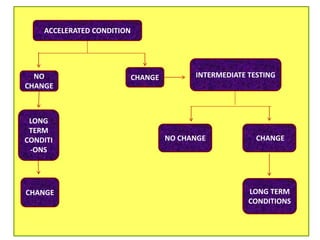
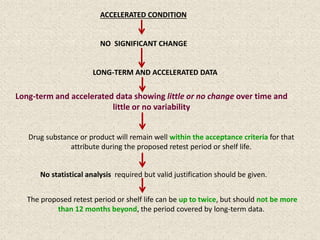
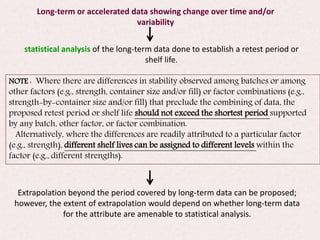
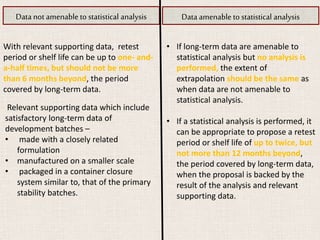
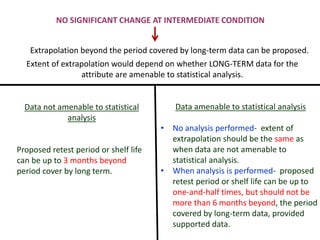
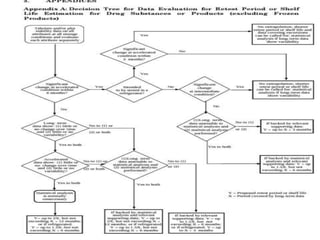
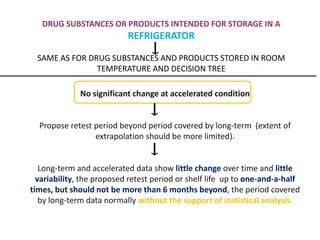
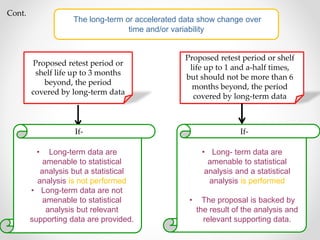
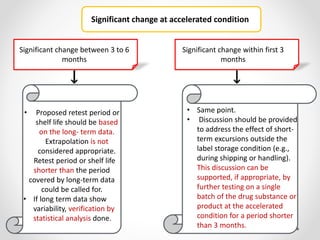
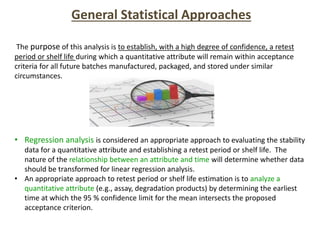
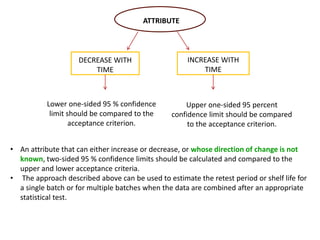
This document provides an overview and guidelines for evaluating stability data to estimate retest periods or shelf lives for drug substances and products. It discusses general principles, data presentation, extrapolation, and statistical approaches. Evaluation methods are described for products stored at room temperature or below room temperature. The document also includes a decision tree outlining steps for data analysis, extrapolation considerations, and factors that influence proposed retest periods.