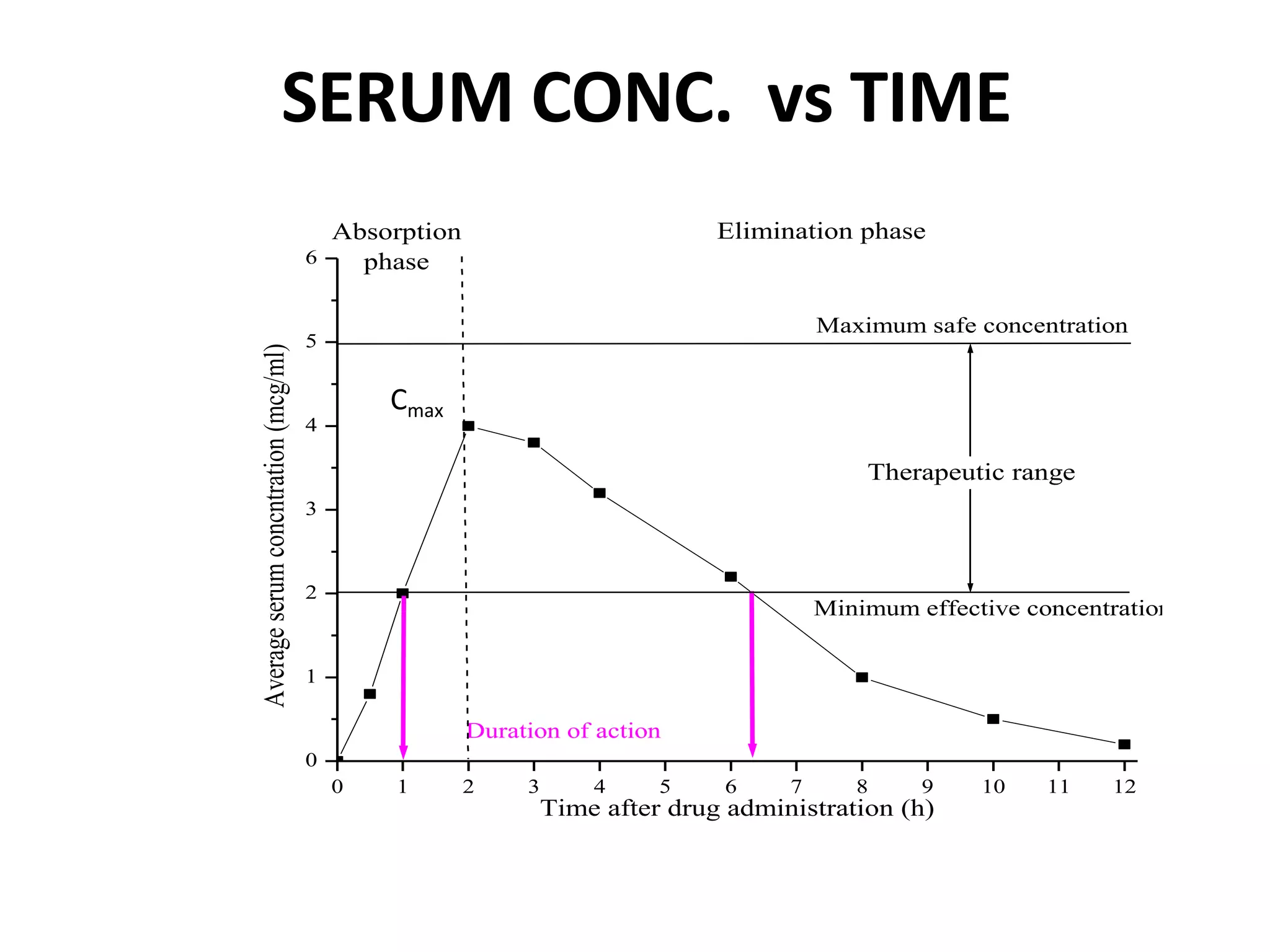
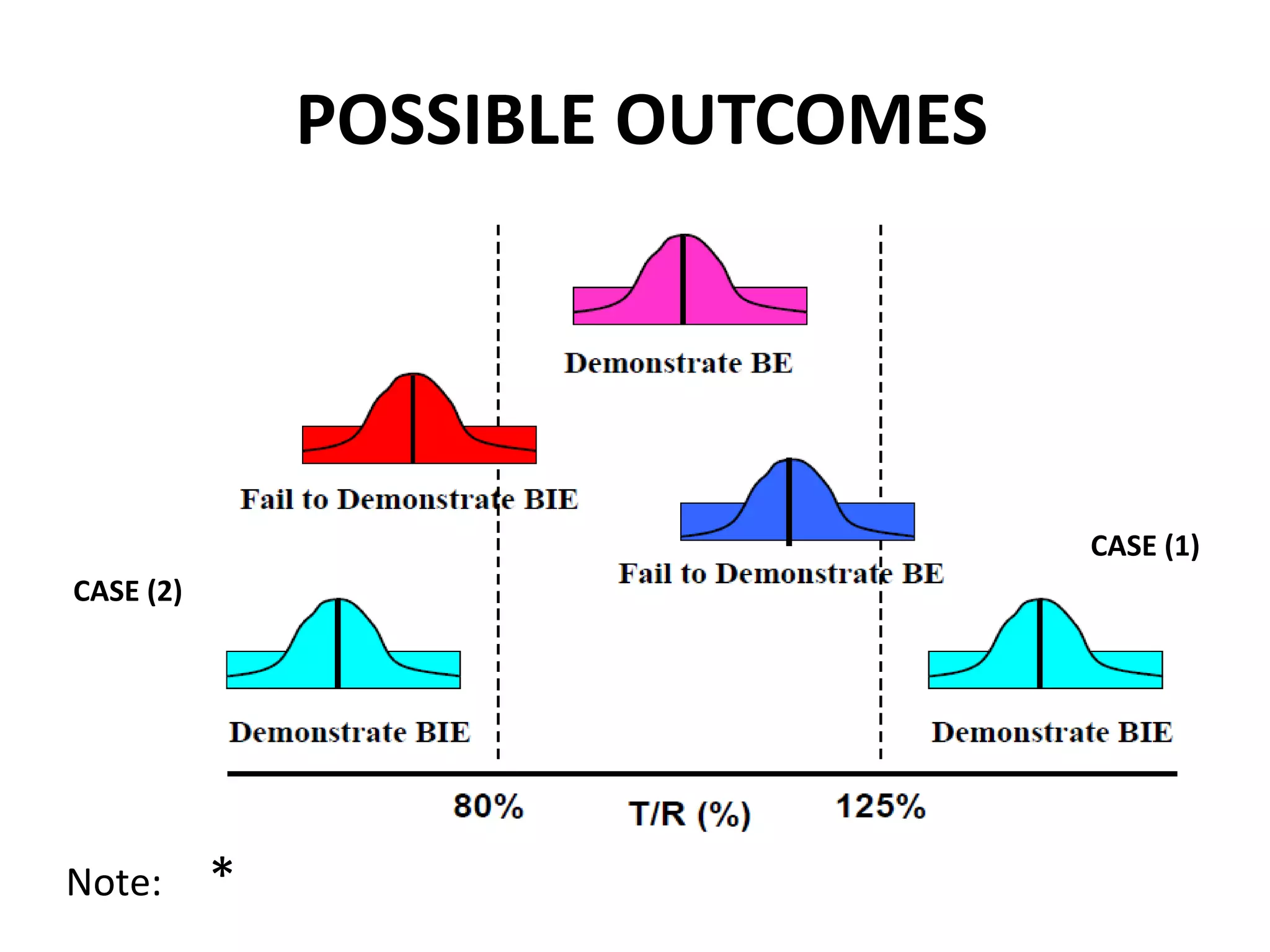
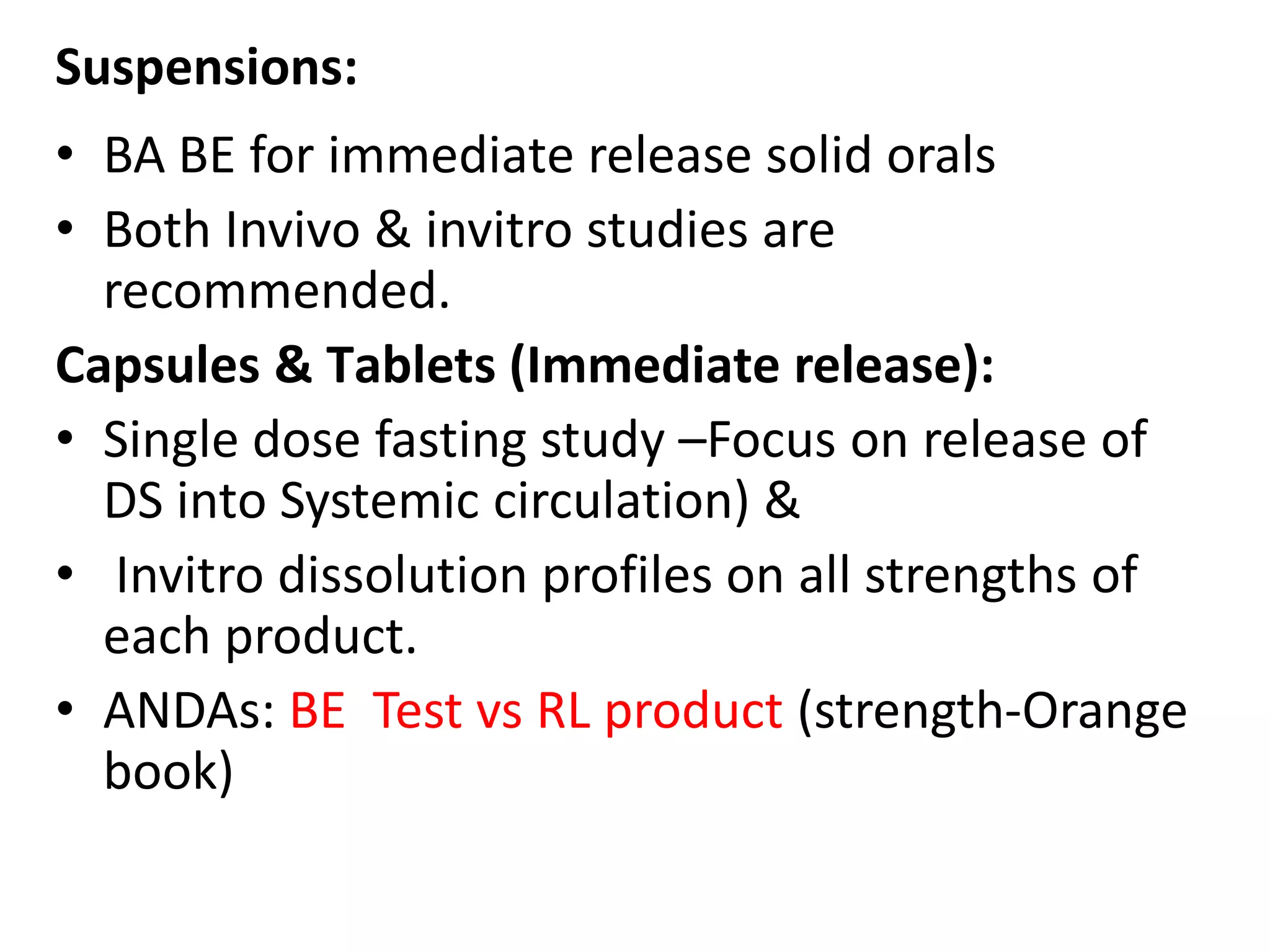
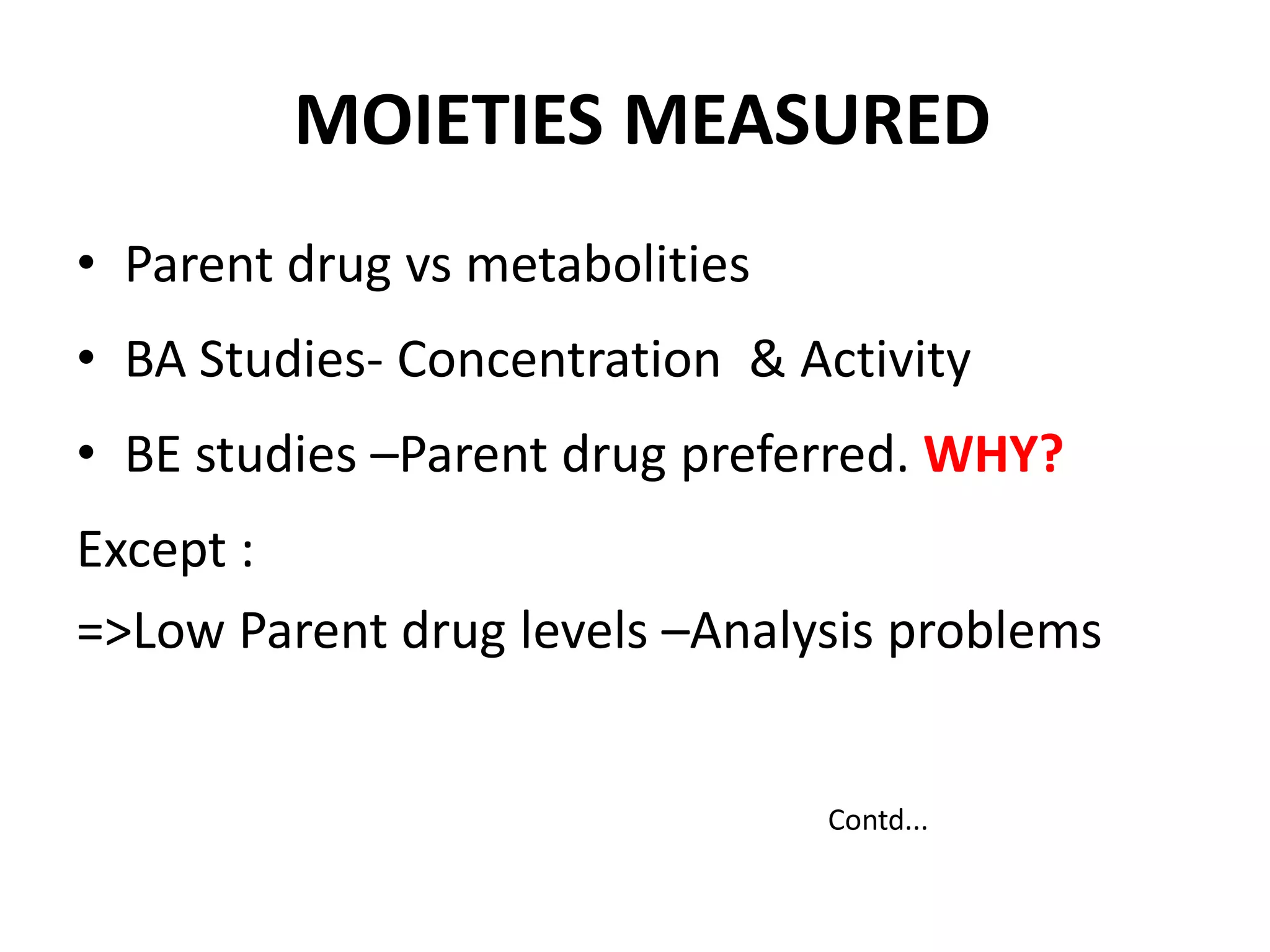
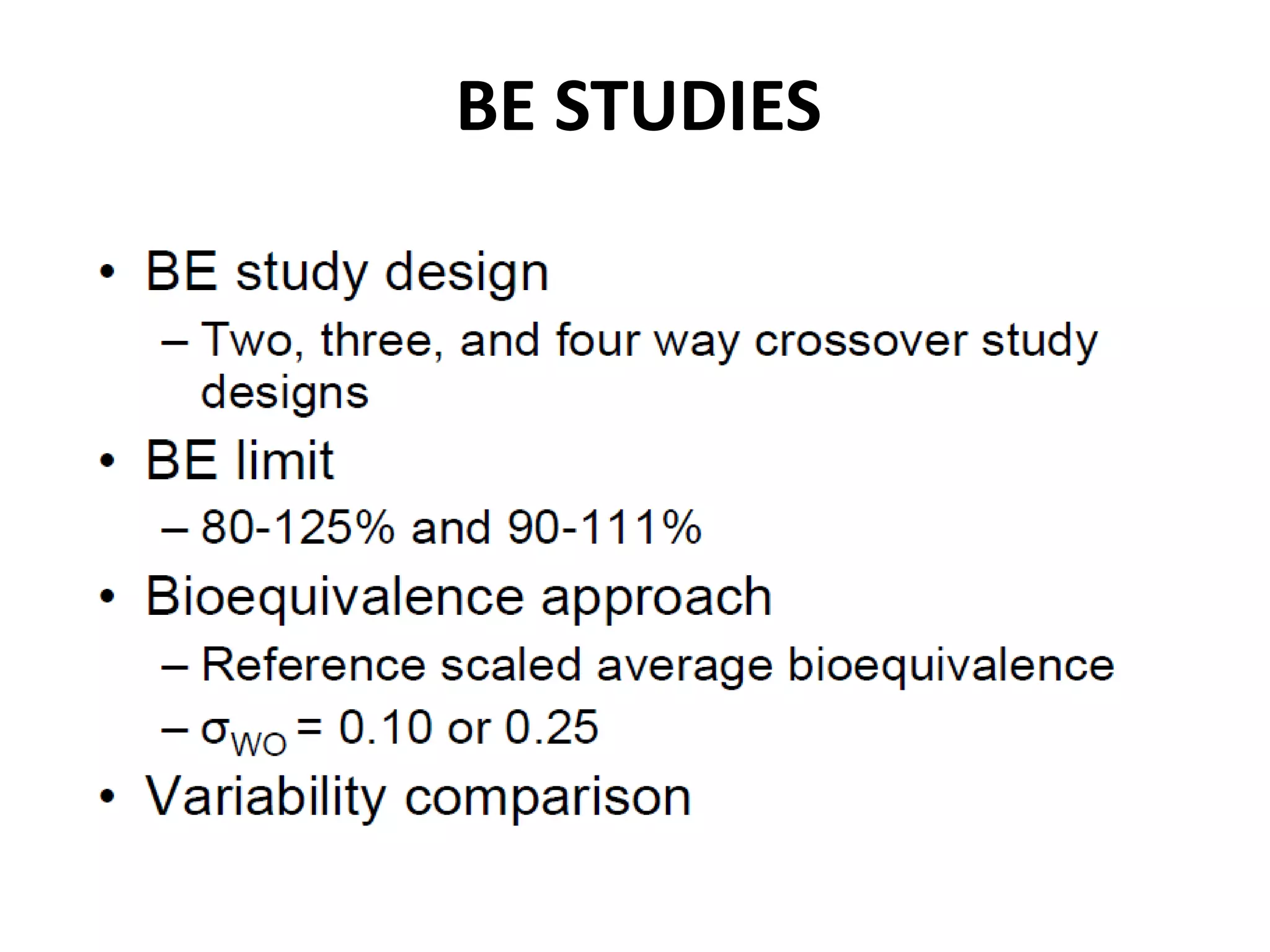
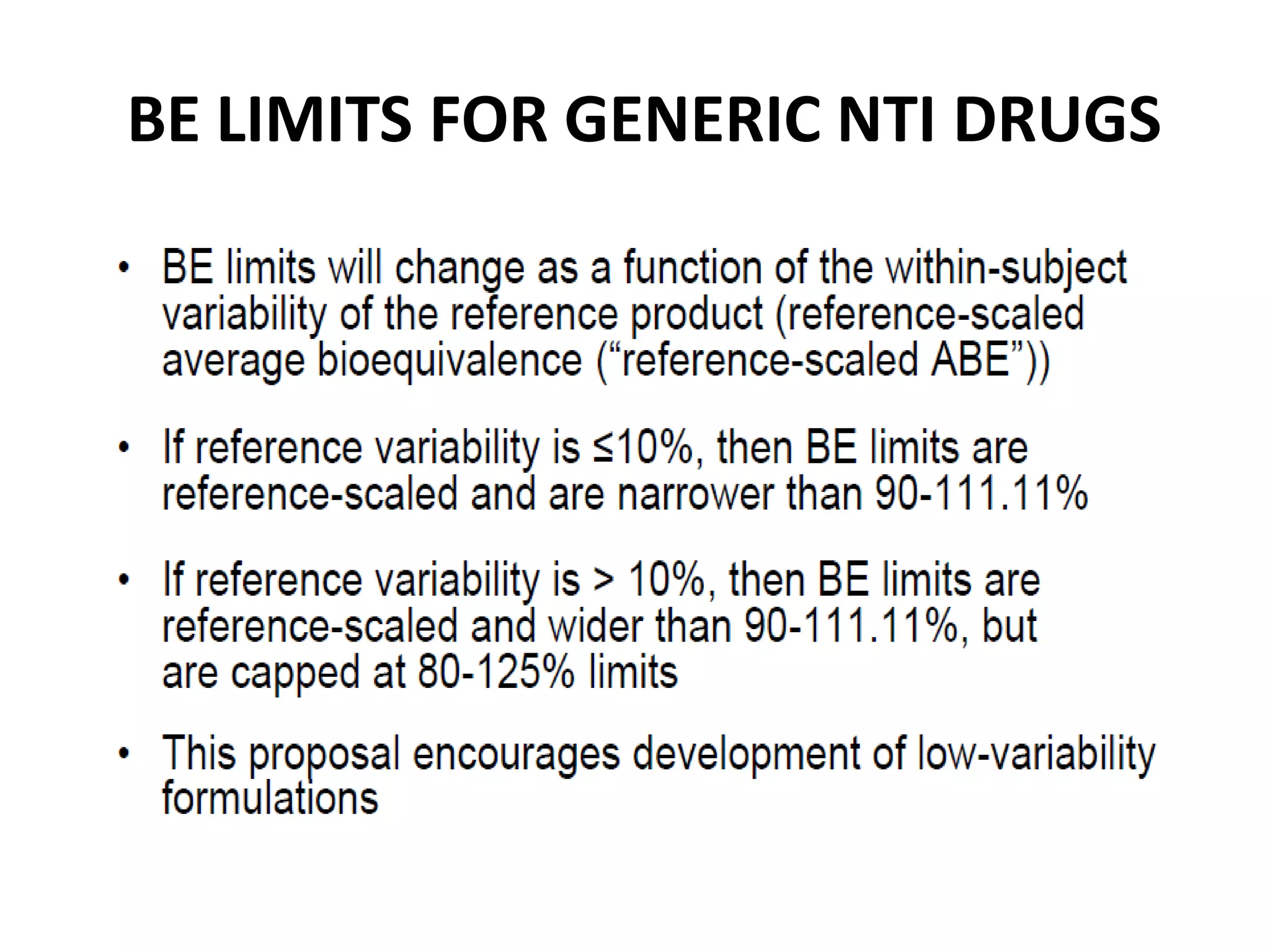
The document discusses bioavailability (BA) and bioequivalence (BE) studies for orally administered drugs, outlining regulatory considerations, study methods, and documentation requirements. It emphasizes the importance of demonstrating that drug formulations used in clinical trials are safe and effective, while also addressing specific cases for waivers and methodologies for measuring BA and BE. The content is structured around FDA guidelines, aiming to ensure therapeutic equivalence between generic and reference drug products.