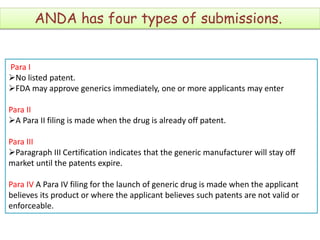
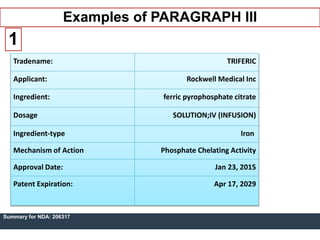
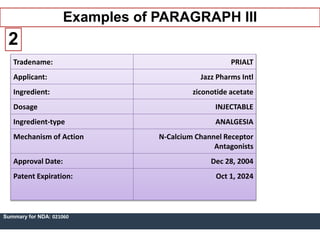
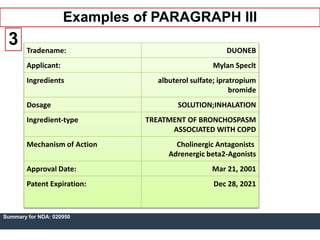
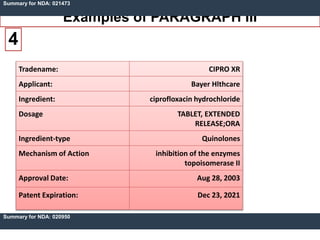
An ANDA contains data submitted to the FDA to review and approve a generic drug product. Once approved, a generic manufacturer can produce and market a safe, low-cost alternative to the innovator drug. All approved drugs are listed in the Orange Book. ANDAs have four types of submissions: Paragraph I for drugs with no listed patents allowing immediate approval; Paragraph II for off-patent drugs; Paragraph III where generics agree not to market until patents expire; and Paragraph IV for generics believing patents are invalid allowing earlier market entry. The examples show drugs with Paragraph III certifications and their patent expiration dates.