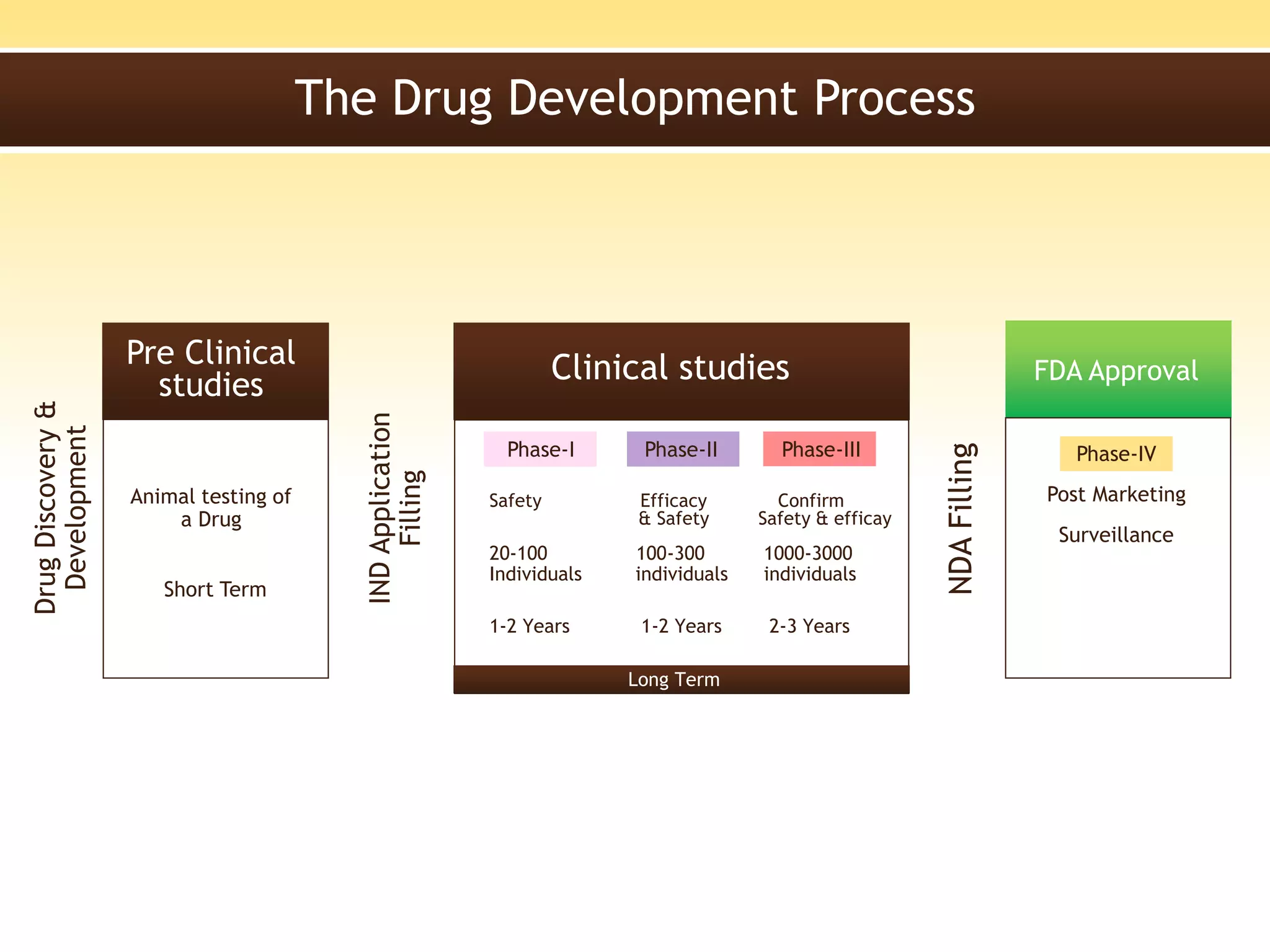
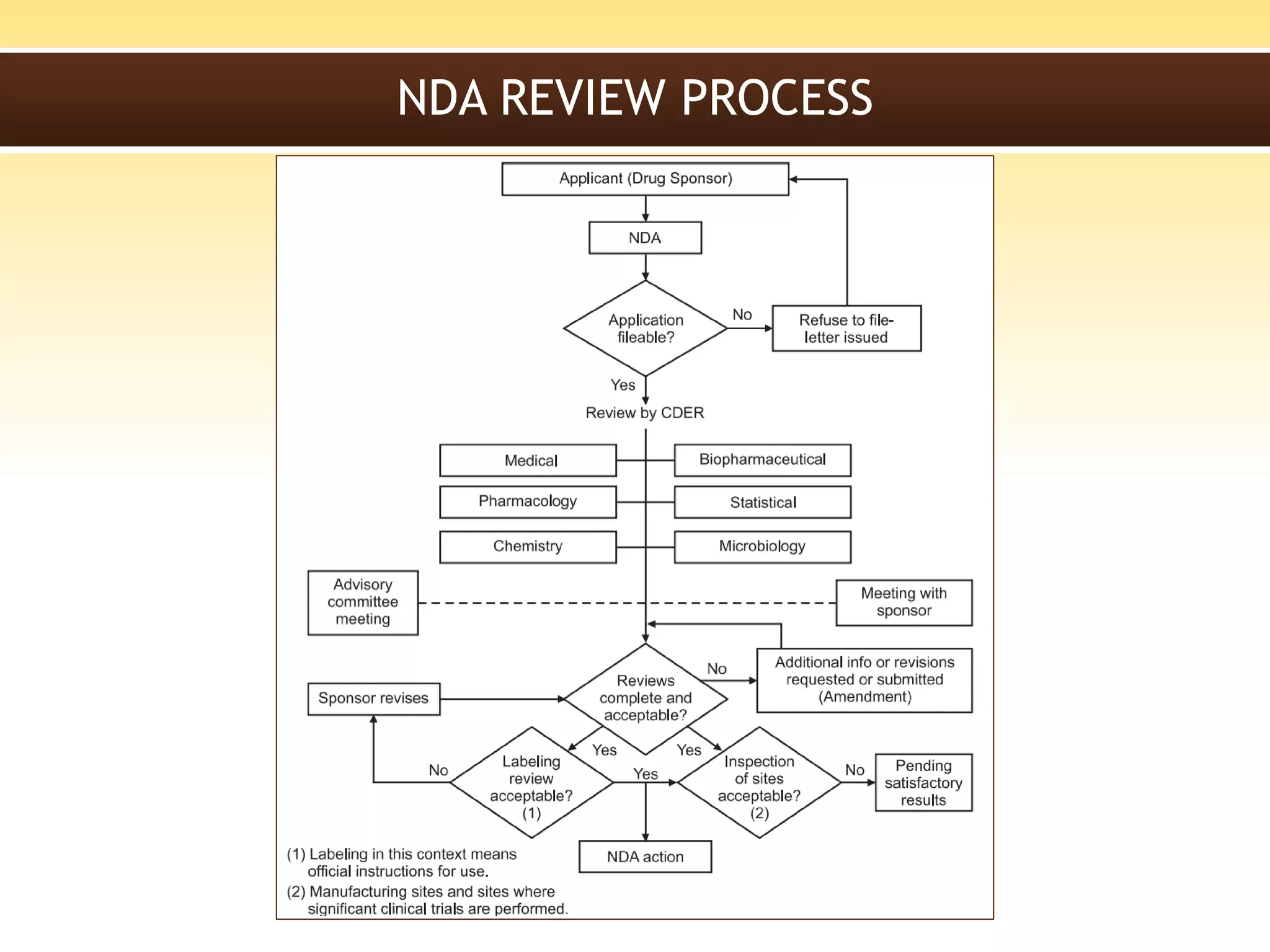
A New Drug Application (NDA) is a request submitted to regulatory authorities, such as the FDA, for approval to market and sell a new pharmaceutical drug, including data from clinical studies and information on safety, efficacy, and manufacturing. The NDA must include 20 sections detailing various aspects of the drug, and is essential for establishing the drug's safety and effectiveness before it becomes available to the public. The review process by the FDA includes a preliminary assessment and can lead to either standard or accelerated review timelines.