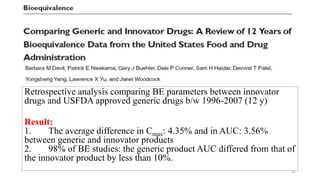
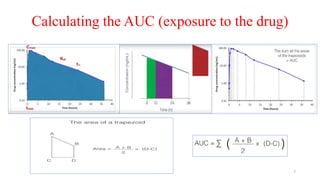
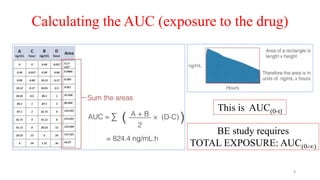
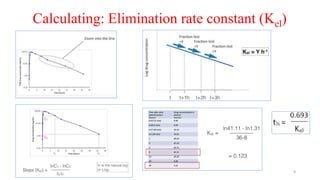
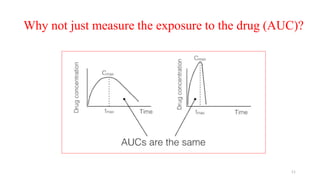
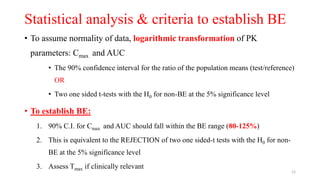
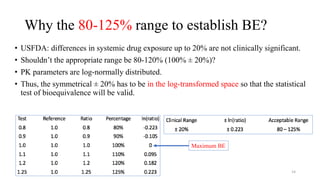
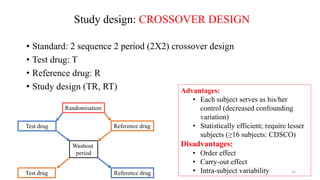
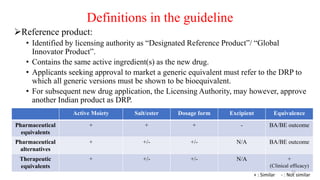
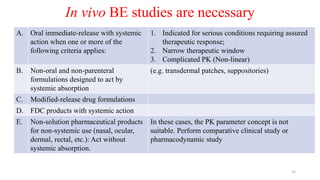
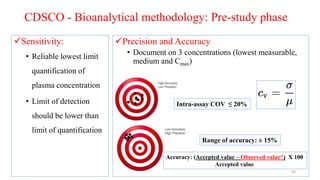
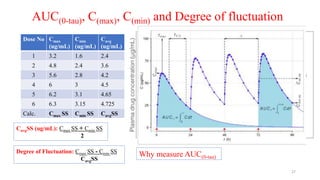
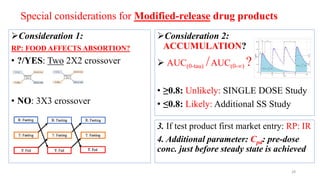
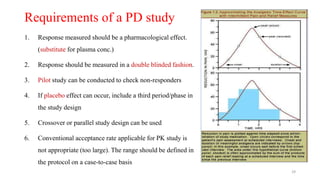
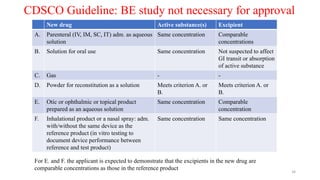
The document discusses bioavailability (BA) and bioequivalence (BE) studies, outlining their definitions, significance, and methodologies according to CDSCO guidelines. It details requirements for conducting PK studies, different study designs, analysis criteria, and exceptions based on therapeutic window and drug characteristics. The conclusion emphasizes the importance of BE for generics in improving patient access to affordable medications while maintaining quality.







![PK study: Study design: Other designs
1. REPLICATE CROSSOVER STUDY DESIGN
• For highly variable drugs
• Study design (TRTR, RTRT)
• Minimum no. of subjects: 24 (USFDA)
• Allows comparisons within subject variances
2. PARALLEL STUDY DESIGN
• For drugs with long half-lives
• Administer each treatment to a separate group of subjects with similar demographics
• USFDA: Measure truncated AUC [eg. AUC(0-24)]: PK simulations show it to be an
equally effective parameter.
Period 1 2 3 4
Group 1 T R T R
Group 2 R T R T
17](https://image.slidesharecdn.com/babestudies-180804095517/85/Bioavailability-and-Bioequivalence-Studies-17-320.jpg)









![What about Biopharmaceuticals
• Vaccines, , blood components, gene therapies, tissues, monoclonal antibodies
• TERM: Biosimilar
• Establishing biosimilarity
1. Animal study
2. A clinical study or studies [including the assessment of immunogenicity and
pharmacokinetics (PK) or pharmacodynamics (PD)] that are sufficient to demonstrate
safety, purity, and potency in 1 or more appropriate conditions of use for which the
reference product is licensed and for which licensure is sought for the biosimilar product.
CDSCO guidelines available:
http://www.cdsco.nic.in/writereaddata/Proposed%20Guidelines%20for%20Similar%20Biologic%202016.pdf 35](https://image.slidesharecdn.com/babestudies-180804095517/85/Bioavailability-and-Bioequivalence-Studies-35-320.jpg)