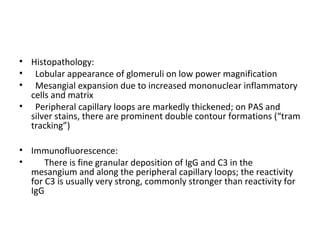
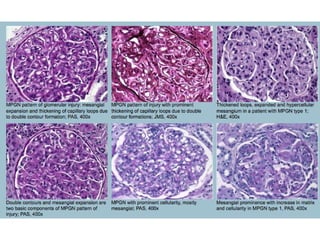
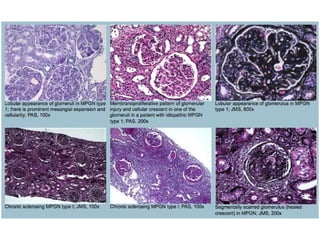
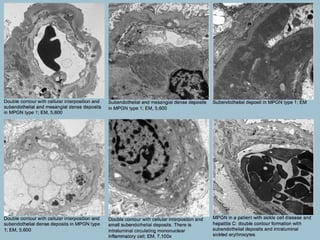
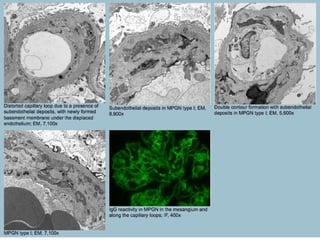
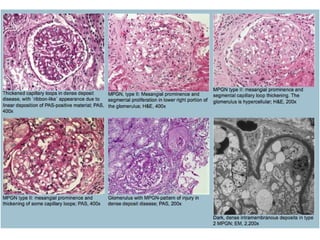
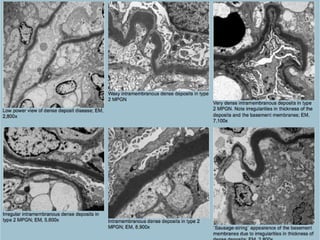
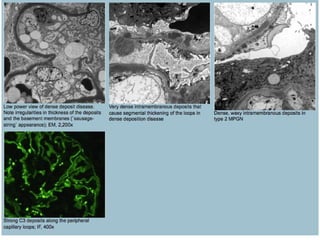
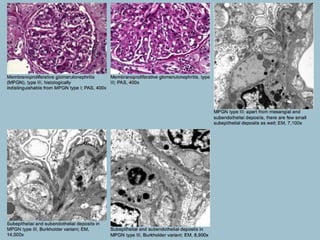
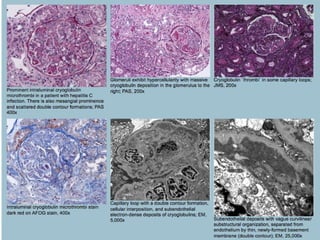
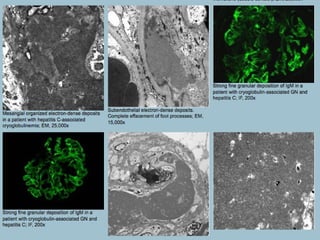
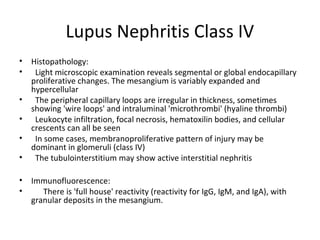
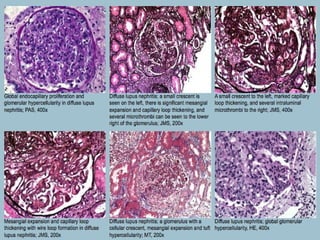
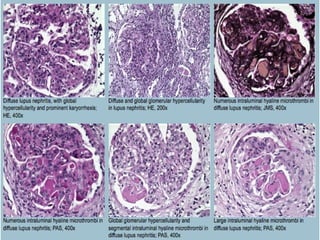
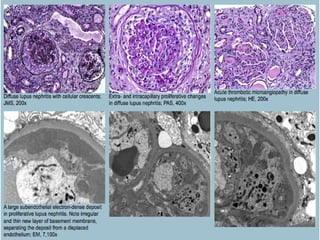
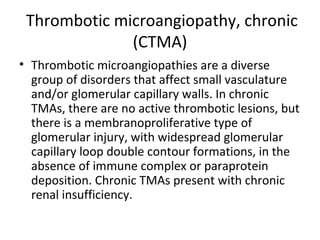
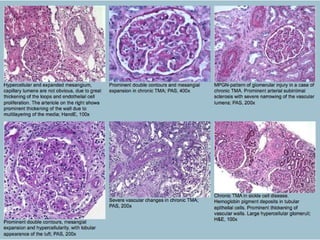
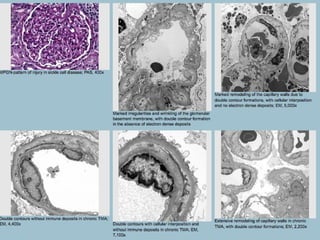
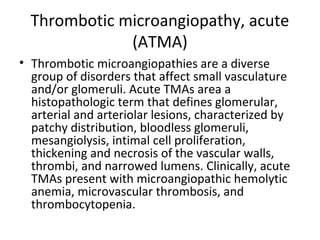
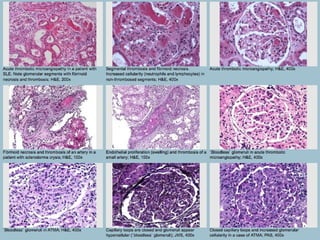
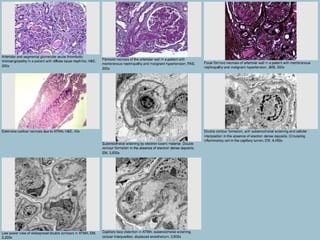
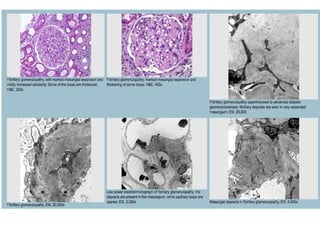
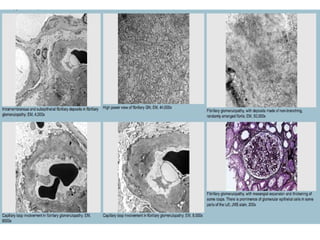
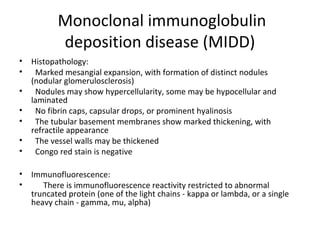
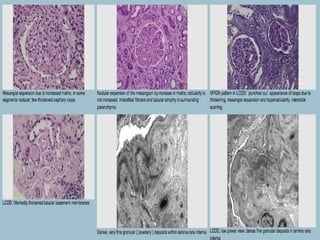
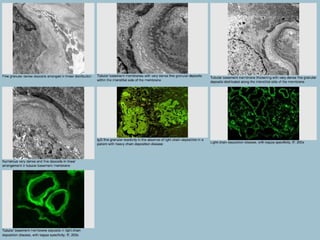
This document discusses several types of glomerular diseases that present with a membranoproliferative pattern of injury on histology, including membranoproliferative glomerulonephritis (MPGN) types I, II, and III, cryoglobulin-associated glomerulonephritis, lupus nephritis class IV, and thrombotic microangiopathies (both acute and chronic). It provides details on the etiology, clinical features, histopathology, immunofluorescence, and electron microscopy findings of each condition.