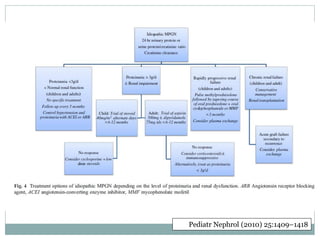
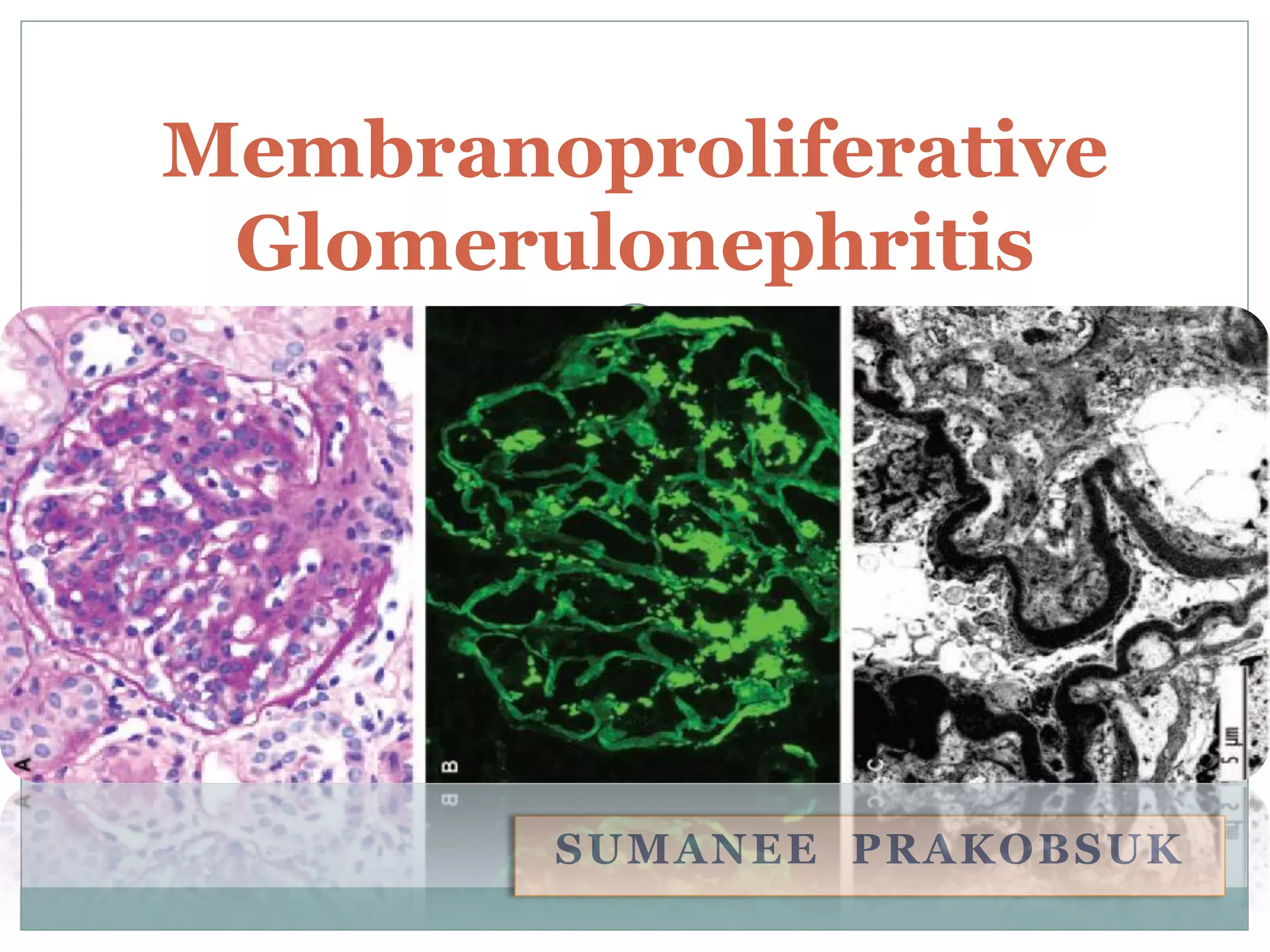
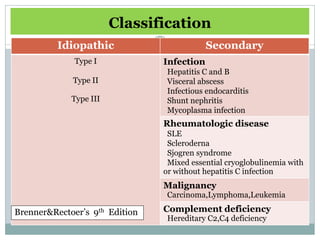
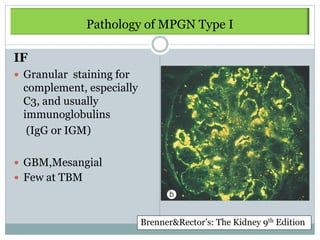
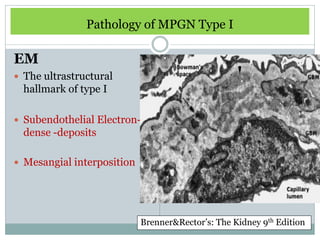
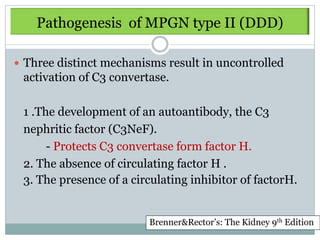
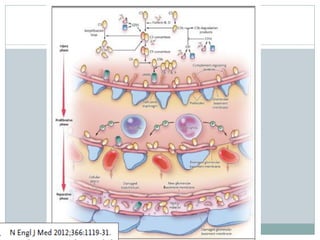
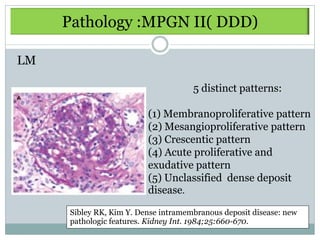
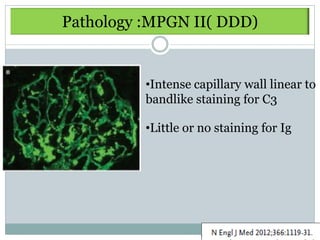
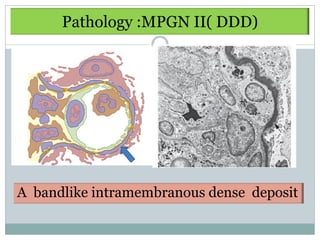
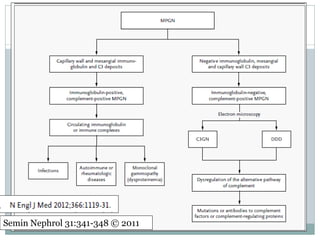
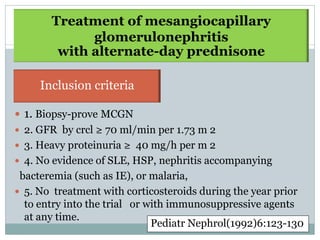
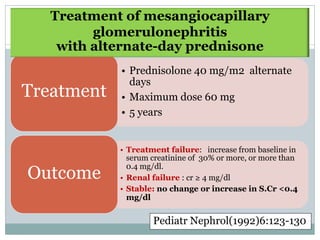
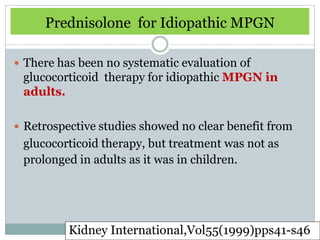
Membranoproliferative glomerulonephritis (MPGN) is a type of glomerulonephritis characterized by thickening of the glomerular capillary walls. It accounts for 7-10% of biopsy-confirmed glomerulonephritis cases. MPGN is classified into three main types based on electron microscopy findings of immune complex deposition. Type I has subendothelial deposits, Type II has dense deposits in the glomerular basement membrane, and Type III has both subendothelial and subepithelial deposits. Treatment involves addressing any underlying causes and may include immunosuppressive agents like corticosteroids and cytotoxic drugs. A randomized control trial found alternate-day pred







![Immunosuppressive drugs
Level of Author Design N Rx Duration Results/Comments
Evidence [Rx : C]
1
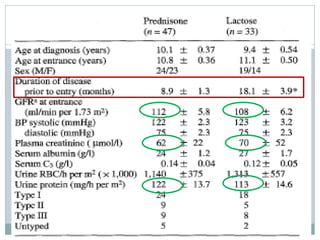
Tarshish RCT 80
[47/33]
Pred 40 mg/m2 130 mo Children only, predomly
MPGN I, stable renal fn
AD vs pcb
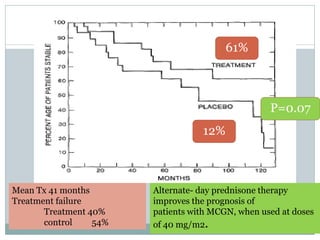
Pred 61% [Rx] vs 12% [C]
No difference found,
1
Cattran RCT 59 CY + coumadin 18 mo
[27/32] + dipyridamole mixed MPGN I > II
CY vs No Rx
1985
3 Strife UCT 17 Pred 2 mk AD 2y MPGN III only, nephrotic range
[16/1] did worse, 3/16 dvled RF
3 Davis CT 27 Pred+IS [NS] - No effect
[19/8]
3 Orlowski UCT 50 P/AZA/CP/chlorambucil 79 mo 10 y F/U, ↓ Uprot c triple drug
in combi Rx
3 Ford UCT 19 PO/IV pred 2 mk + 8-10 wk, Children; 6.5 y F/U, early Rx,
ACEI then x2-3 shorter course,
y tapered ↓ glom prolif
dose
3 Faedda UCT 19
IV/PO CY+P 10 mo 15/19 remission, 7 y F/U
1994 [different
combinations]
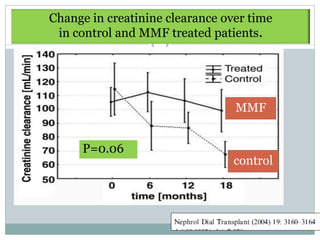
Adeera Levin., Mx of MPGN; Evidence based recommendations; KI 1999; 55: S41-S46](https://image.slidesharecdn.com/mpgnpam-120514124515-phpapp01/85/MPGN-Pam-64-320.jpg)
![Antiplatelet
Level Author Design Rx Durati Results/Comments
of evi N [Rx : C] on
1
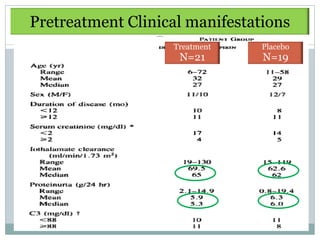
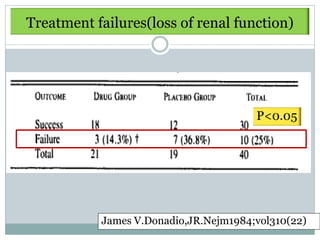
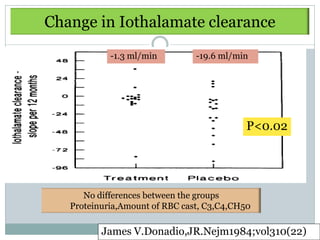
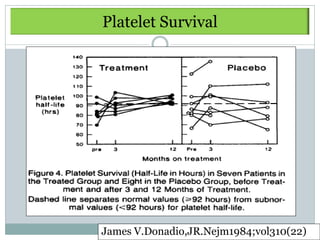
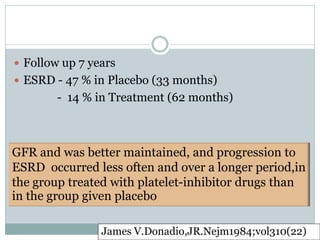
Donadio
1984
RCT Dipyridamole
75mg/d + ASA 325
12 mo -Tx : significantly delay
rate of GFR ↓
Prospective mg/d vs Pcb
[Sig difference at 7 y]
40 [21/19]
-No change in Uprot,
hematuria, Complement
-Mild bleeding complication
required discontinuation 15%
1
Zimmer
man
RCT Warfarin [INR 1.5-2]
+ dipyridamole [75-100
12 mo
[2 y
Prot restriction & HTN control
standardized.
Crossover mg qid] vs Pcb study] Sig reduction in proturia
18 [8/10]
NS diff in renal fn
Significantly ↓ Uprot
1
Zauner
1994
RCT ASA 500 mg/d +
dipyridamole 75
36 mo
[1 g Tx vs 8 g control] [Sig]
18 [9/9] mg/d
PR : Tx 7/10 vs Control
[I 15 + III [both : prot restriction 2/8
3] & HTN control]
-Comment : short F/U
SCr 1.8, Uprot 7 g/d
Adeera Levin., Mx of MPGN; Evidence based recommendations; KI 1999; 55: S41-S46](https://image.slidesharecdn.com/mpgnpam-120514124515-phpapp01/85/MPGN-Pam-65-320.jpg)
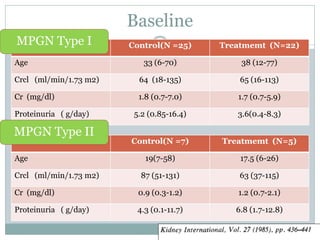
![Evidence based recommendations : KI 1999
Idiopathic MPGN
24 h Uprot & CCr
<3 g >3 g
Normal renal function Abn renal function Normal renal function Abn renal fn
Grade C; Grade A; Grade B;
Child : trial of steroid x 3 Child : trial of Adult : trial of ASA
mo [AD; IV or PO 1 mk] steroids 40 mg/m2 325 mg/d &
Or AD x 6-12 mo Dipyridamole 75-100
Adult : no Rx, observe mg tid x 6-12 mo
F/U q 3 mo;
BP, Lipid monitoring
-No change : continued
F/U, ↓ frequency
- Increase cr or
proteinuria Rx
Adeera Levin., Mx of MPGN; Evidence based recommendations; KI 1999; 55: S41-S46](https://image.slidesharecdn.com/mpgnpam-120514124515-phpapp01/85/MPGN-Pam-66-320.jpg)