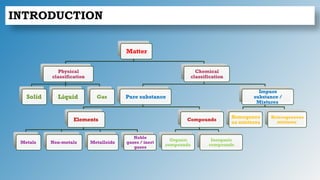
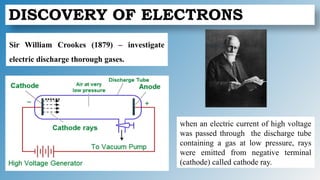
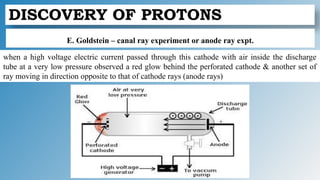
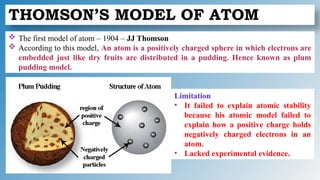
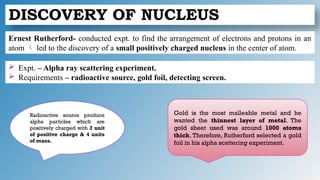
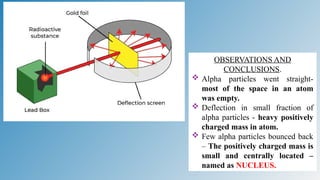
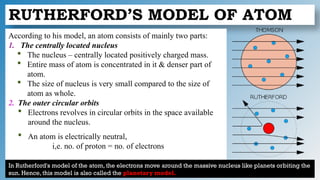
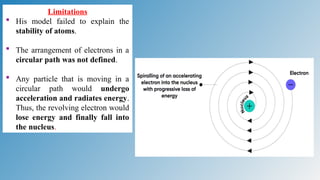
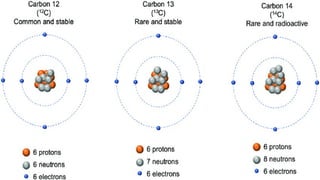
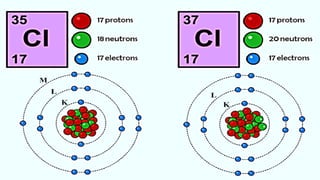
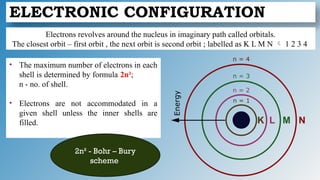
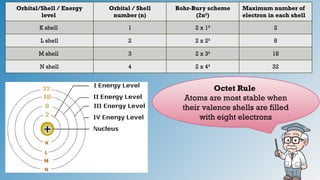
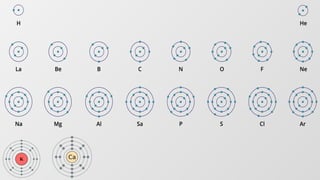
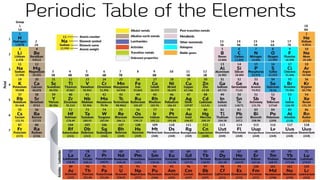
The document discusses the fundamentals of atomic structure, detailing the historical development of atomic theory from ancient philosophers to modern scientists. It covers the discovery of subatomic particles (electrons, protons, neutrons) and models of the atom, including Thomson's Plum Pudding Model, Rutherford's Planetary Model, and Bohr's Atomic Model. Additionally, it explains concepts like isotopes, electronic configuration, and valency.









![VALANCY & VARIABLE VALANCY
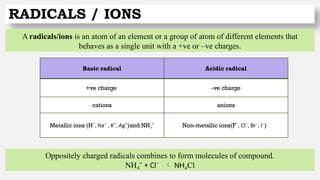
Valancy is the combining capacity of an element or a radicle.
Elements have more than one valency which is known as variable valency.
An element exhibits two different +ve valencies -
Suffix “OUS”- lower valancy
Suffix “IC”- higher valancy
Metal Radical Valency
Iron (Fe)
Ferrous [Fe(II)] – Fe²⁺ 2
Ferric [Fe(III)] – Fe³⁺ 3
Mercury (Hg)
Mercurous [Hg(I)] - Hg⁺ 1
Mercuric [Hg(II)] - Hg²⁺ 2
Tin (Sn)
Stannous [Sn(II)] - Sn²⁺ 2
Stannic [Sn(IV)] – Sn⁴⁺ 4](https://image.slidesharecdn.com/atomicstructure-240824065959-635ac636/85/Atomic-structure-electronic-configuration-29-320.jpg)
