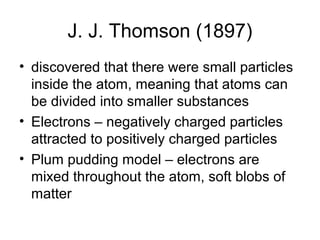
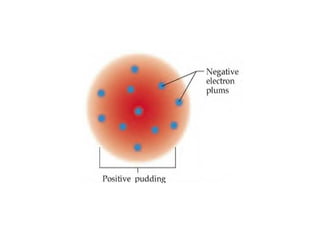
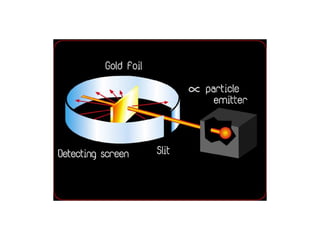
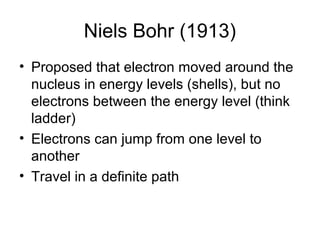
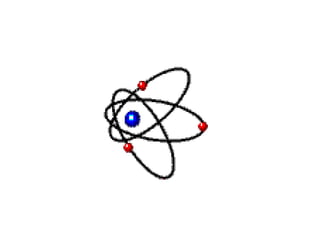
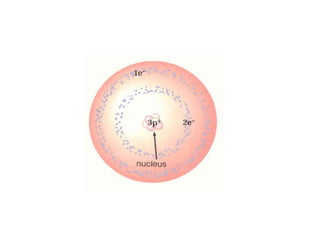
Atomic theory has evolved over time as scientists have discovered more about the internal structure of atoms. Atoms are now understood to be the smallest particle of an element that retains the properties of that element. Atoms contain a nucleus of protons and neutrons surrounded by electrons. The number of protons determines the element, and atoms of the same element have the same number of protons but may vary in the number of neutrons forming different isotopes. Forces such as electromagnetic force hold the structure of the atom together.