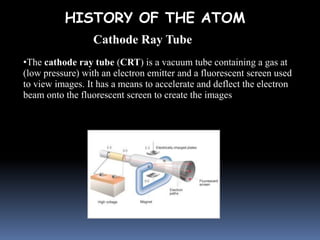
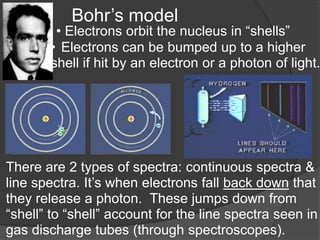
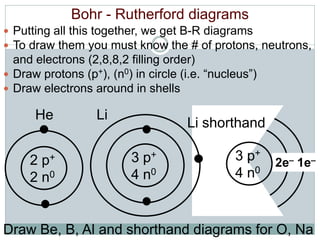
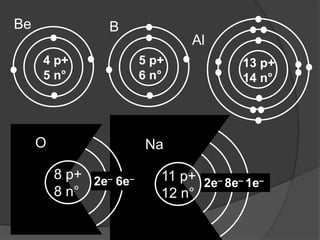
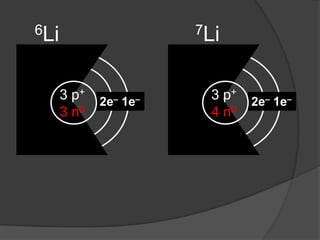
- Aristotle proposed that matter was made of four elements: earth, fire, water, and air. This theory persisted for over 2000 years despite being incorrect.
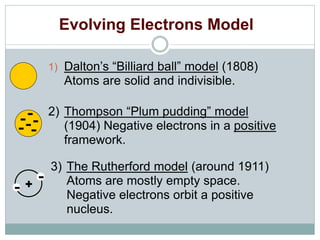
- In the early 1800s, Dalton proposed his modern atomic theory which stated that all matter is made of atoms, atoms of the same element are identical, and atoms combine in fixed ratios.
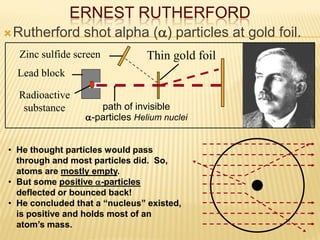
- Rutherford's gold foil experiment in the early 1900s showed that atoms have a small, dense nucleus containing positive charge, overturning the plum pudding model of atoms.