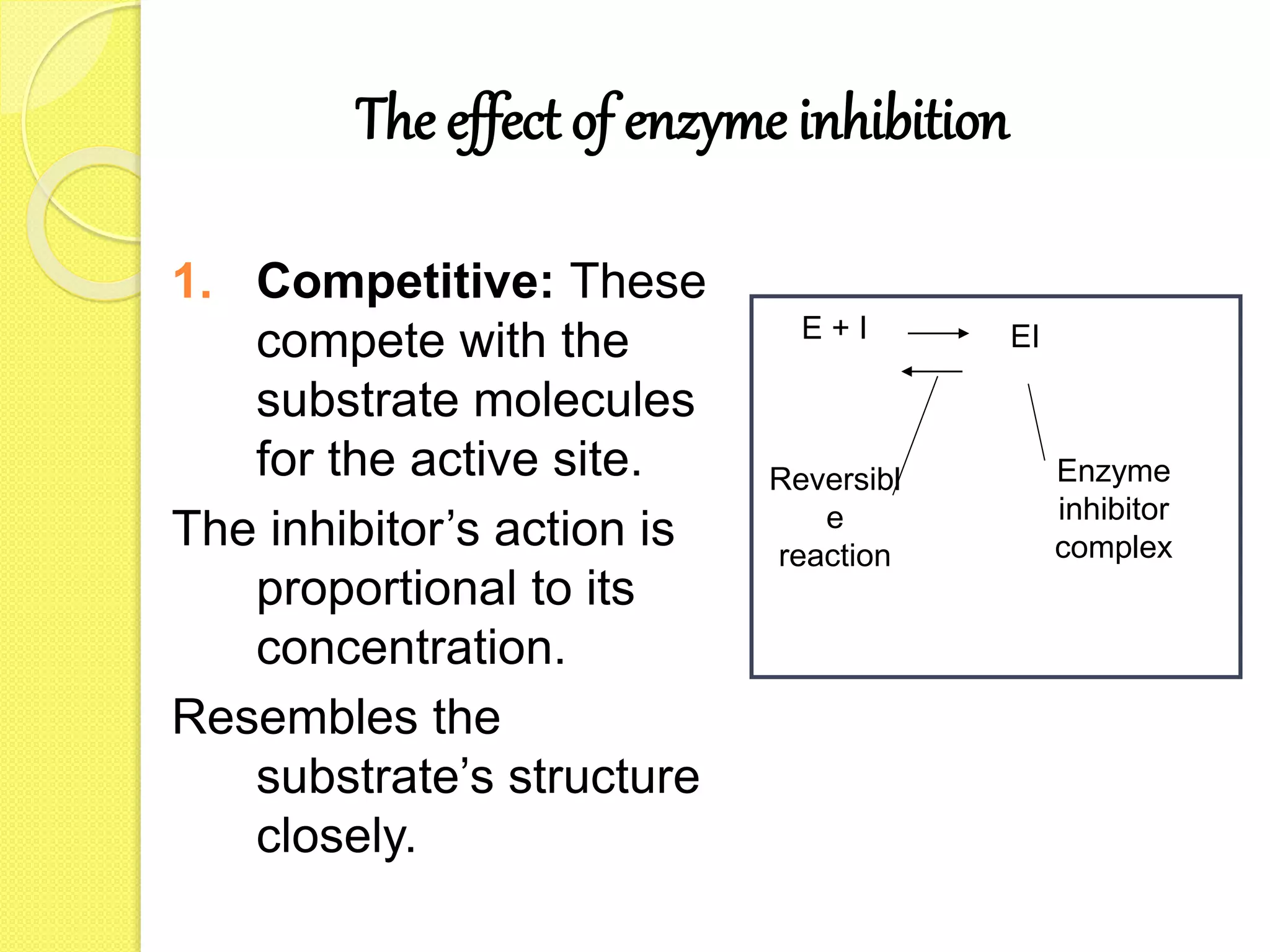
This document discusses coenzymes, cofactors, and enzyme inhibition. It defines cofactors as non-protein compounds required for enzyme biological activity, and divides them into organic and inorganic groups. Coenzymes are loosely bound cofactors that assist enzyme functioning and transport chemical groups between enzymes. Many coenzymes are related to vitamins. Enzyme inhibitors can be competitive or non-competitive, and examples are given of their medical and poison applications.