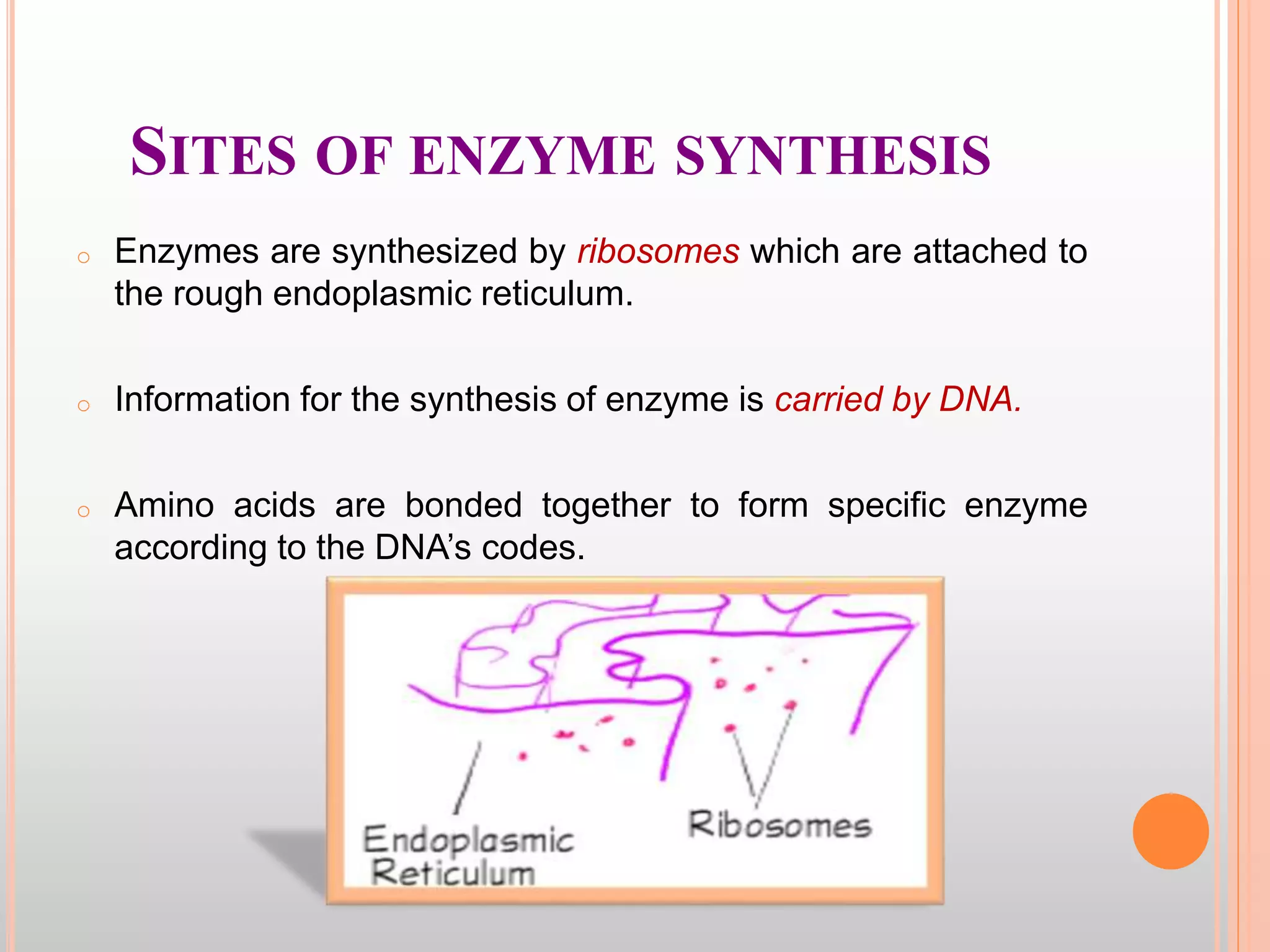
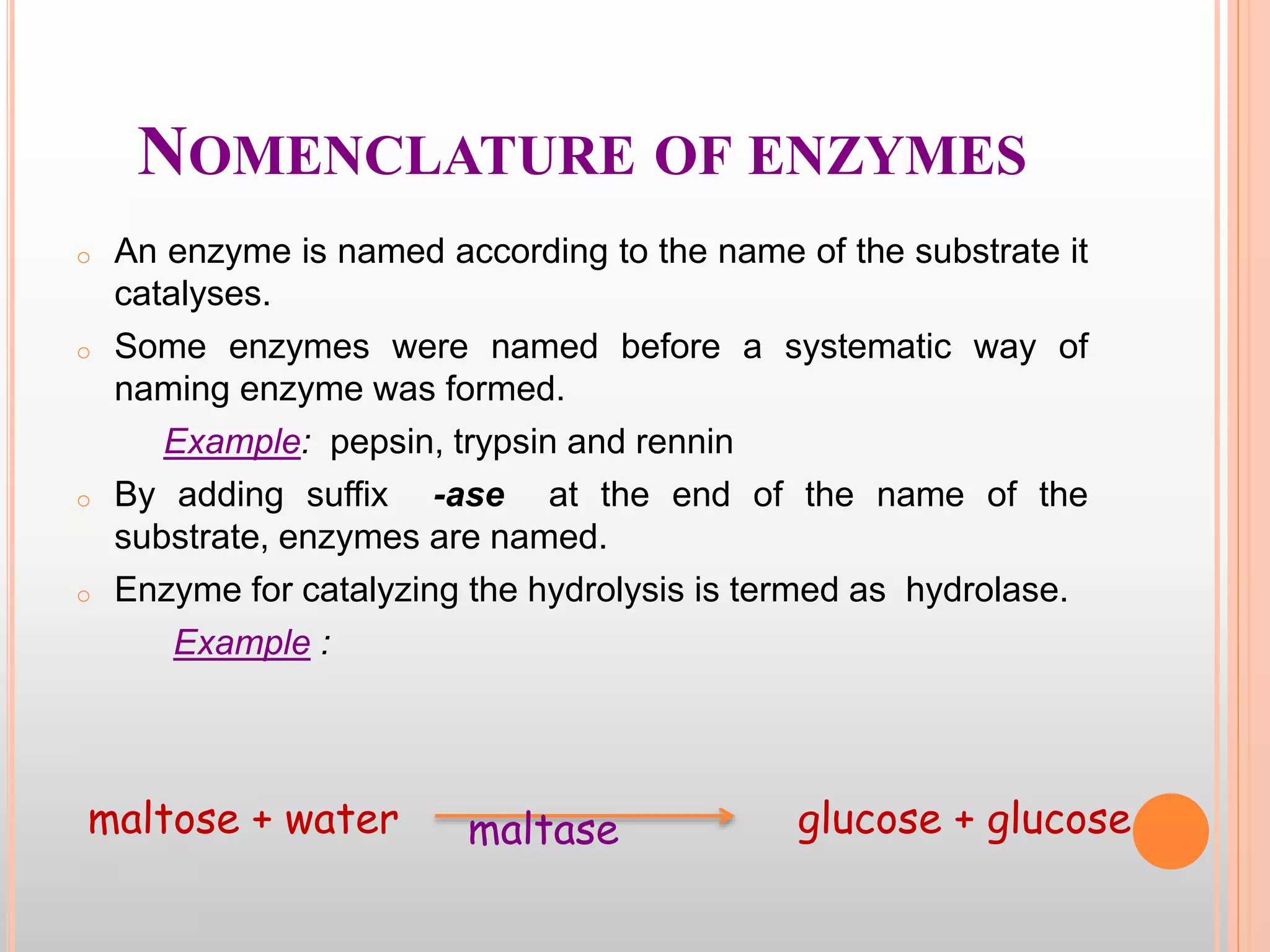
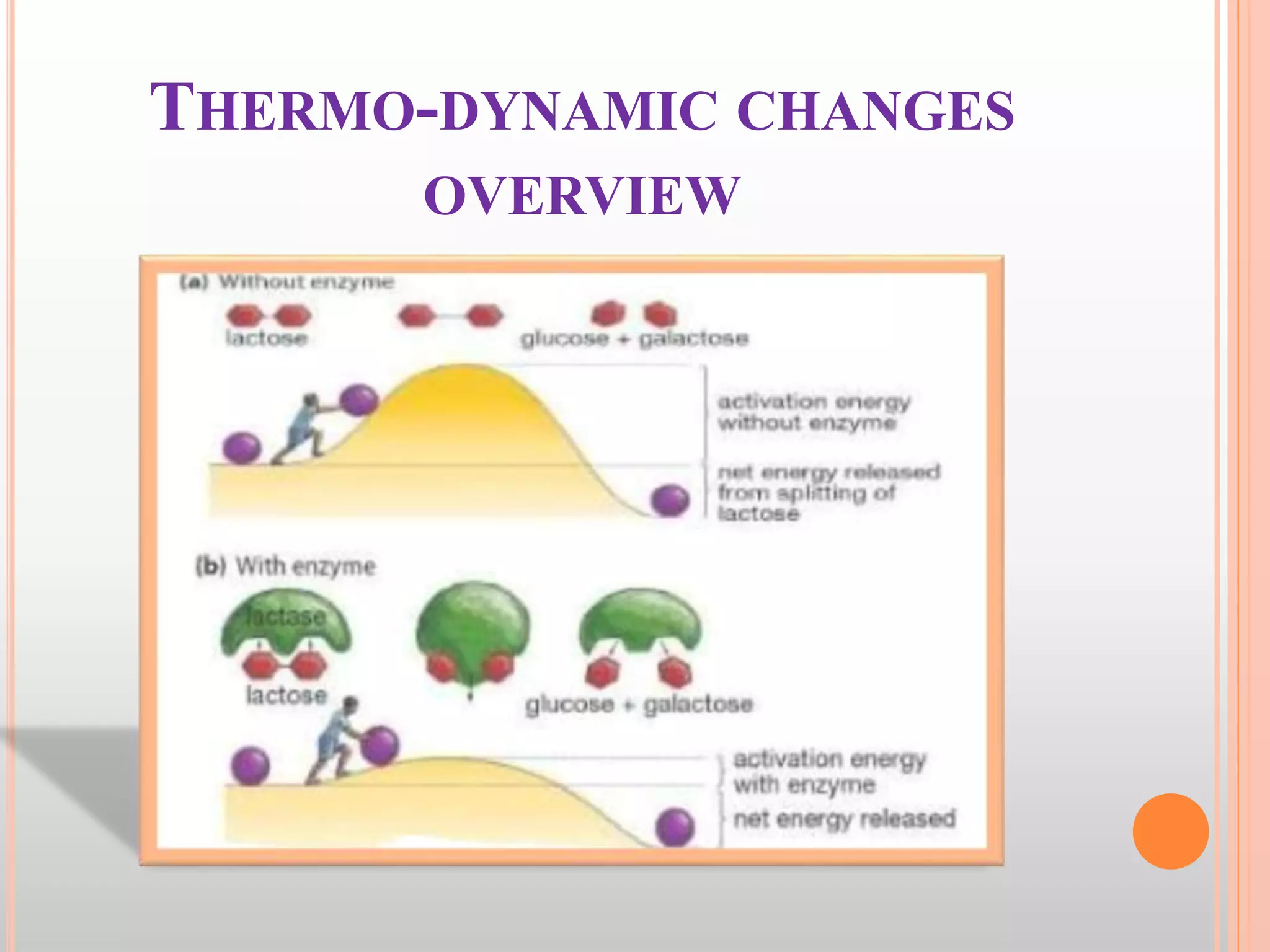
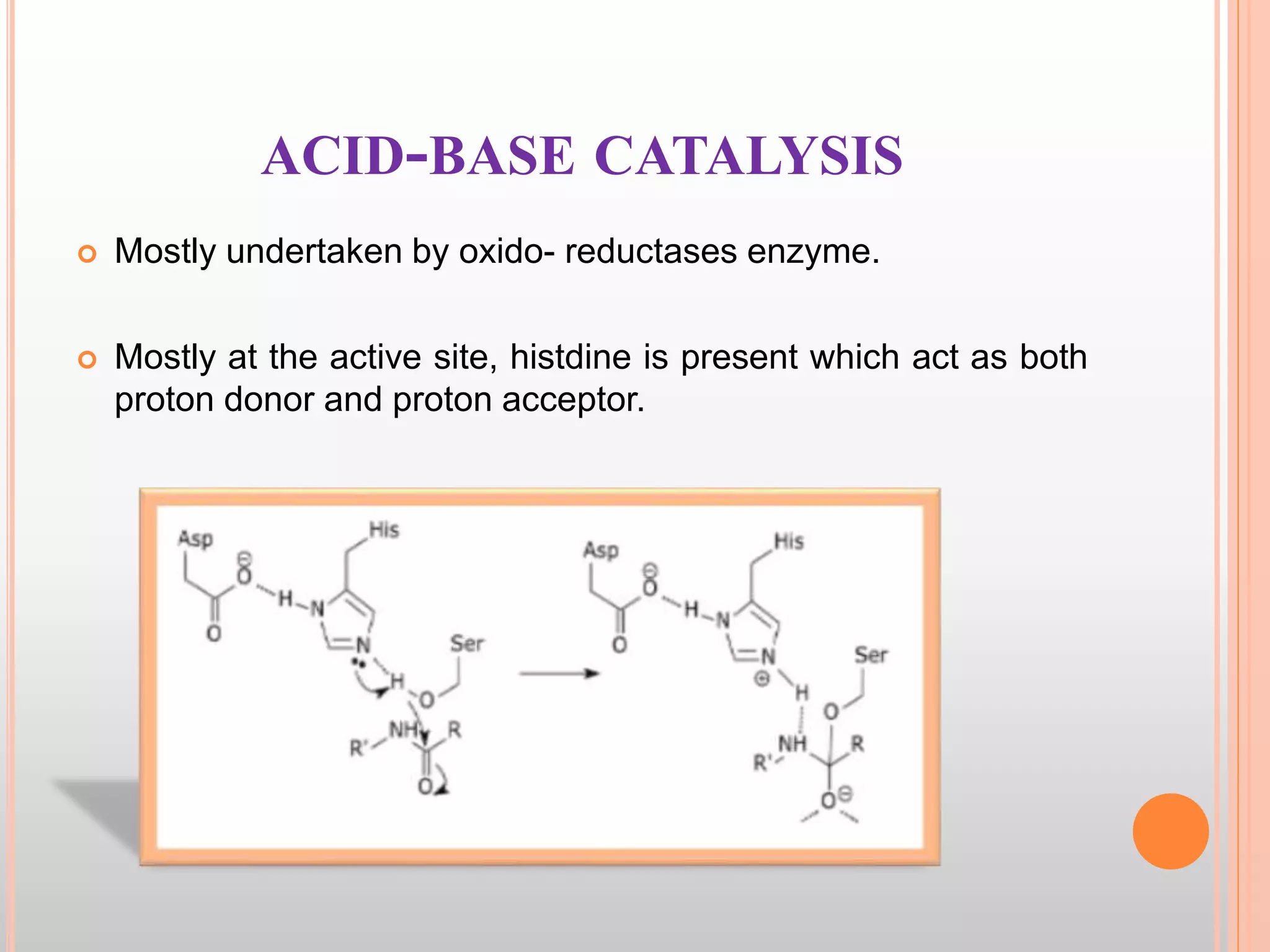
The document provides an overview of enzymes, detailing their chemistry, classification, and mechanisms of action as biological catalysts. It explains enzyme structure, including active sites, cofactors, and the processes of enzyme synthesis and action, emphasizing their specificity, catalytic efficiency, and factors affecting their activity. Additionally, it describes enzyme kinetics, types of inhibition, activation processes, and the various forms of enzyme specificity.
































![MICHAELIS-MENTEN EQUATION
Michaelis-Menten Equation:
“It is an equation which describes how reaction velocity varies
with substrate concentration.”
Vmax [S]
Vo=
Km+[S]
Where
Vo is the initial reaction velocity.
Vmax is the maximum velocity.
Km is the Michaelis constant = (k₋₁+k₂)/k₁.
[S] is the substrate concentration.](https://image.slidesharecdn.com/enzymes-160501072742/75/Enzymes-40-2048.jpg)


















