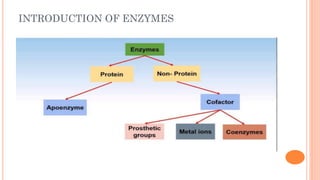
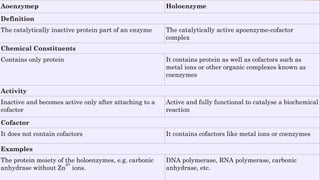
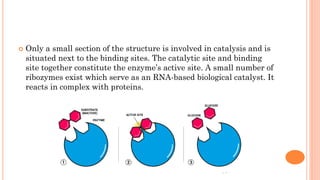
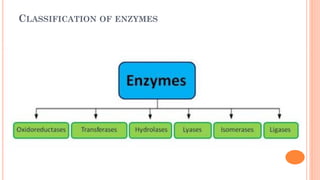
The document provides an overview of enzymes, highlighting their importance as biological catalysts that accelerate biochemical reactions and their classification into different types such as oxidoreductases, transferases, and ligases. It describes the structure of enzymes, including the distinction between apoenzymes and holoenzymes, and discusses the role of cofactors in enzyme functionality. Additionally, it outlines the characteristics of enzymes, their amino acid composition, and the factors affecting their activity.