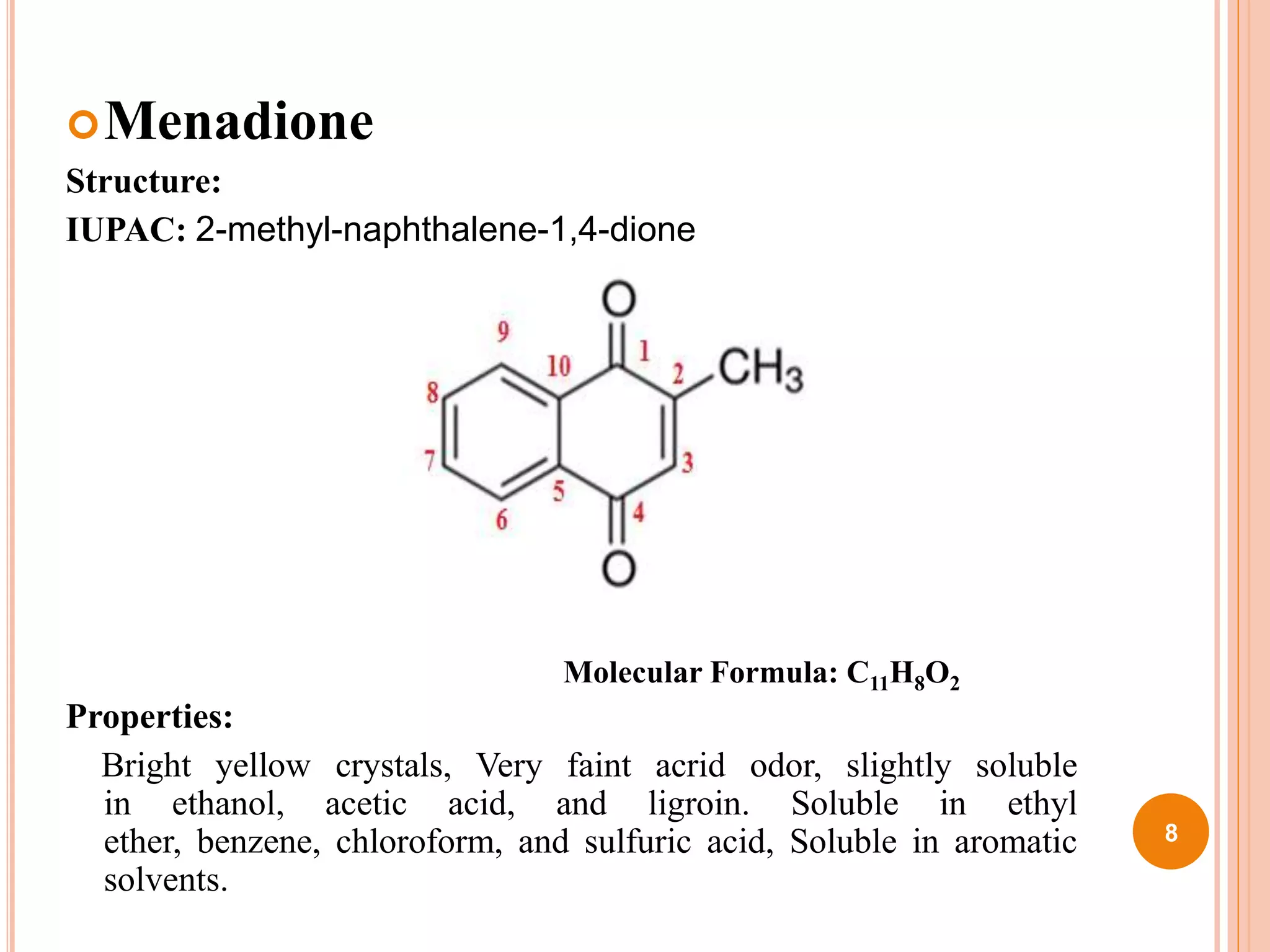
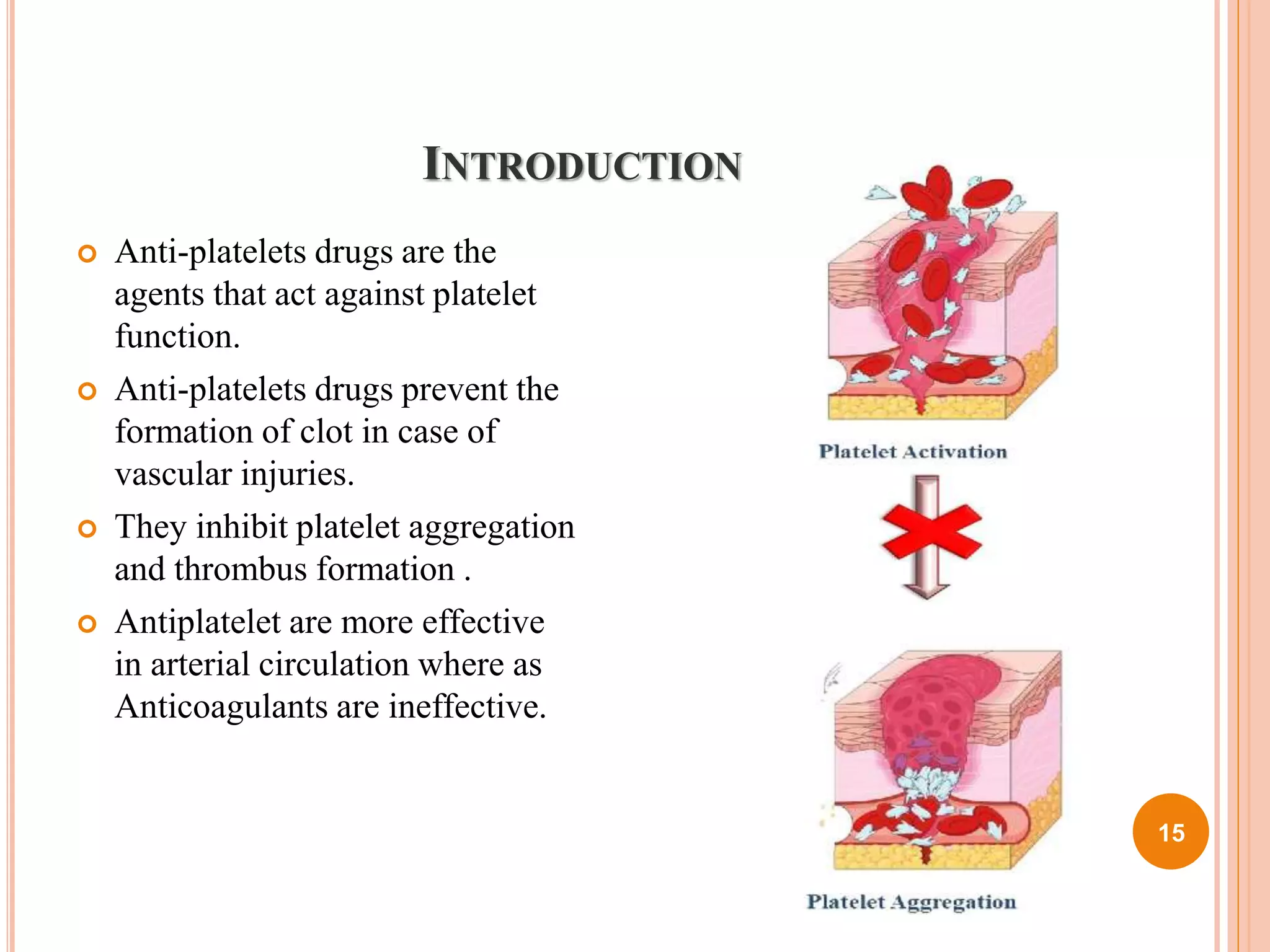
The document discusses coagulant and anticoagulant agents, including their classification, mechanisms of action, and therapeutic uses. It covers specific drugs like vitamin K and warfarin, detailing their structures, pharmacokinetics, adverse reactions, and dosages. Additionally, it provides references for further reading on medicinal chemistry.