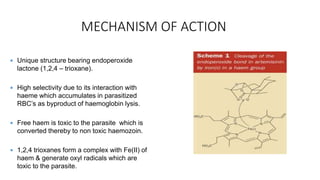
Artemisinin is a drug extracted from the plant Artemisia annua that is highly effective against malaria. It was discovered in the late 1960s by Chinese scientist Tu Youyou as part of Project 523, which screened thousands of herbal remedies to find treatments for malaria. Artemisinin was found to clear malaria infections more effectively than chloroquine in clinical trials. The active compound was isolated in 1972 and named qinghaosu. Artemisinin has a unique chemical structure containing an endoperoxide bridge that is responsible for its antimalarial properties. It acts by damaging heme released from malaria parasites, which the parasites cannot then detoxify. Artemisinin combination therapies are




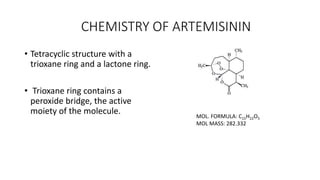
![STRUCTURE ACTIVITY RELATIONSHIP
Presence of a ‘Trioxane’ moiety which
consists of the Endoperoxide & Doxepin
oxygens that is evidently displayed by a
simplified version of 3- Aryltrioxanes.
Reduction of Artemisinin to
Dihydroartemisinin gives rise to a chiral
centre,that may ultimately lead to the
formation of ‘Prodrugs’ which could either
be oil soluble or water soluble.
Like the simpler Aryltrioxanes the two
prevailing stereoisomers Artemether,
Artesunate which are found to be active.
R
O
O
O
H
OCH3H
ARYLTRIOXANES [R= F or COOH]
O
O
O
O
CH3
H
H3C
CH3
H
OHH
DIHYDROARTEMISININ](https://image.slidesharecdn.com/artemisinin-170223162710/85/Artemisinin-6-320.jpg)