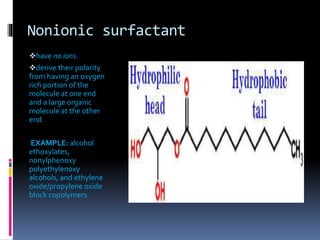
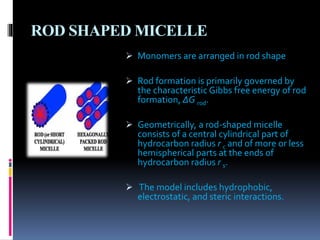
The document discusses the solubilization process, which involves breaking intermolecular bonds and forming interactions between solute and solvent molecules. It describes the role of surfactants, their types (anionic, nonionic, cationic, zwitter), and the formation of micelles, including their types (spherical, rod-shaped, laminar) and the factors influencing their stability. Additionally, it highlights the differing micellization processes in aqueous versus nonpolar environments.