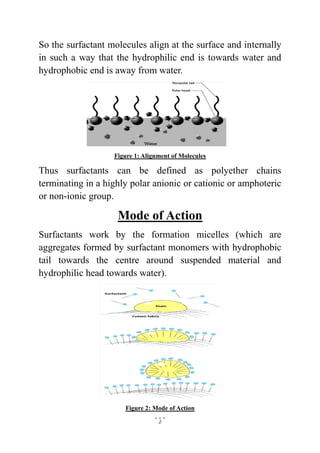
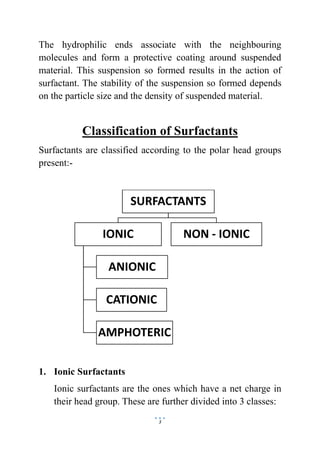
This document discusses surface active agents, also known as surfactants, which are compounds that lower surface tension between two liquids or a liquid and solid. It classifies surfactants based on their structure and polar head groups. Surfactants have a hydrophilic head and hydrophobic tail, which allows them to align at liquid interfaces. They act by forming micelles that suspend materials by coating them with hydrophilic heads facing water. Surfactants are classified as ionic, including anionic, cationic and amphoteric types, or non-ionic based on the charge of their polar head group.