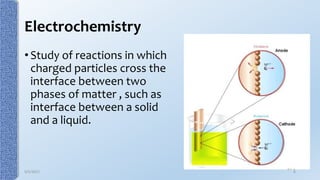
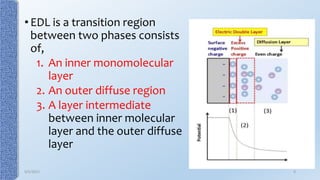
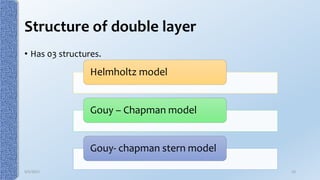
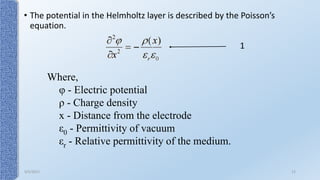
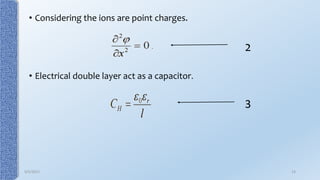
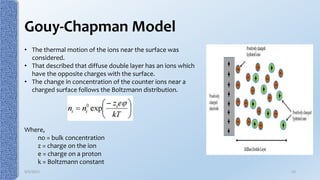
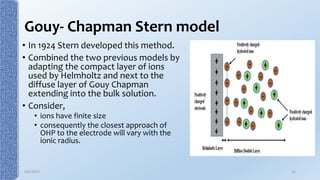
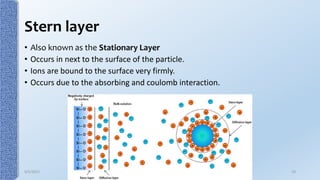
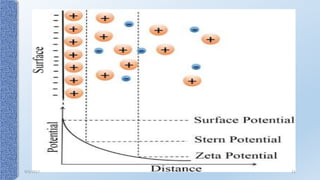
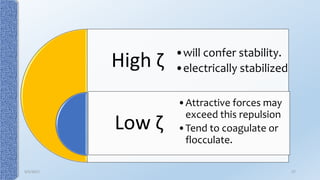
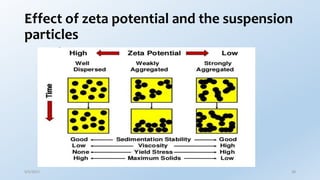
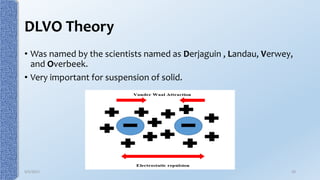
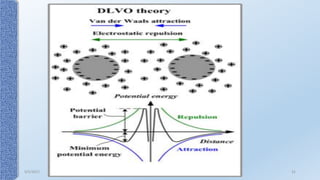
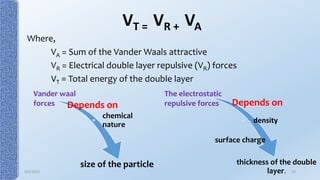
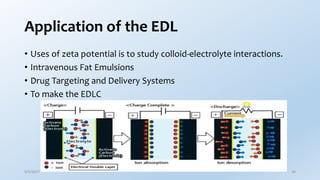
This document provides an overview of the electrical double layer (EDL) theory. It describes the three main models of the EDL structure: Helmholtz model (single layer of ions), Gouy-Chapman model (diffuse ion layer), and Gouy-Chapman-Stern model (combination of compact and diffuse layers). The Gouy-Chapman-Stern model is now widely accepted. It depicts the EDL as consisting of an inner Stern layer and an outer diffuse layer. Applications of EDL theory include measuring zeta potential to determine colloid stability and developing the DLVO theory of colloid interactions and stability.