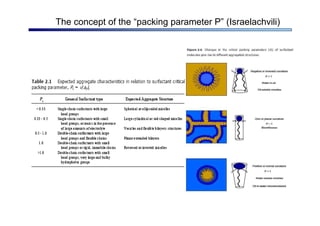
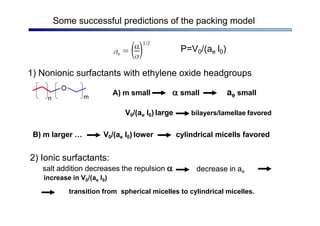
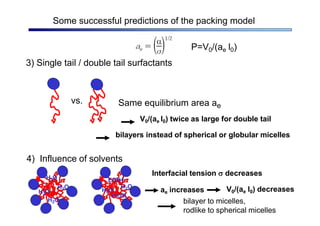
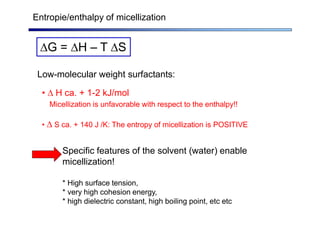
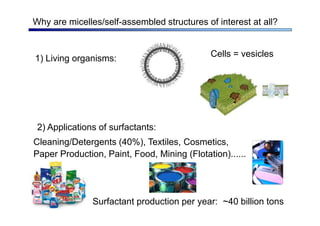
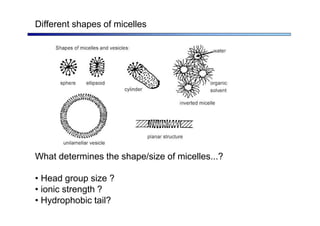
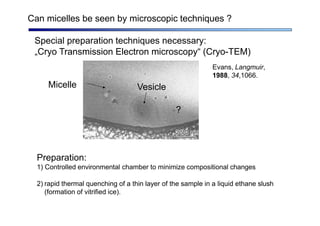
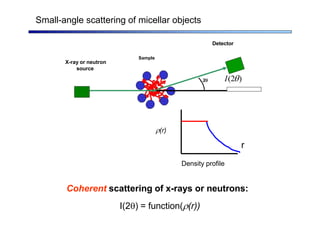
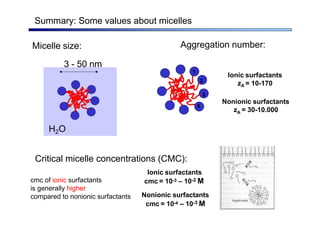
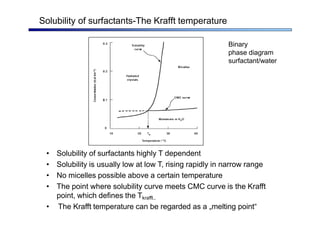
The document discusses micelle formation from surfactants in water. Surfactants are amphiphilic molecules with both hydrophilic and hydrophobic parts that can self-assemble into micelles in aqueous solution above a critical concentration. The packing parameter concept predicts micelle shape based on the surfactant's geometry. Spherical, cylindrical, and bilayer structures are expected for different packing parameter ranges based on the surfactant head group size and tail volume. Micelle characteristics like size and critical concentration can be understood through thermodynamic models balancing hydrophobic effect, interfacial tension, and head group repulsion.



















![aggregation number n dominates
– (when n , phase separation model)
Closed aggregation model
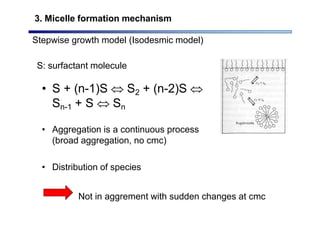
3. Micelle formation mechanism
Kn = [micelles]/[monomers]n = [Sn]/[S]n
CMC = (nKn)-1/n
nS Sn , eq.
cooperative phenomenon!
Kn=1030; n=20
[monomer]
c-[monomer]](https://image.slidesharecdn.com/micelleformation-230801171120-f220c220/85/MicelleFormation-pptx-27-320.jpg)