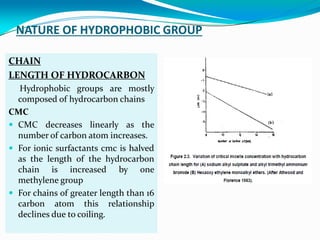
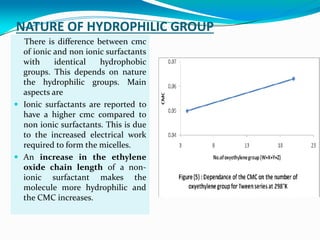
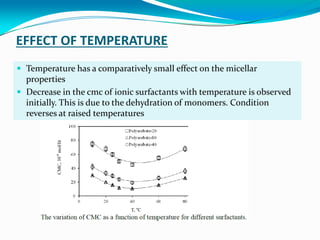
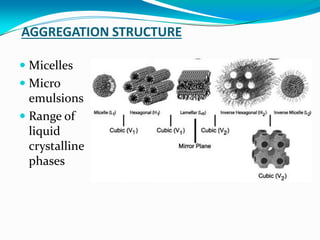
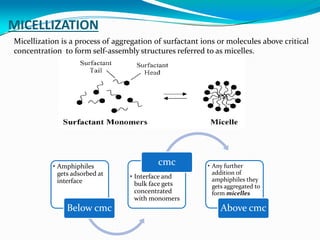
Micellization is the process where surfactant molecules spontaneously self-assemble into aggregates called micelles above a critical concentration known as the critical micellar concentration (CMC). Micelles form to minimize contact between the surfactant hydrophobic tails and water. The CMC and micelle size are affected by factors like the surfactant chain length and head group, counter ion type, temperature, and presence of additives. Micelles can incorporate hydrophobic drugs to improve their solubility and stability for drug delivery applications.


![SURFACTANTS
They are low to moderate molecular weight compounds
with a hydrophobic part and a hydrophilic part
[amphiphile].
Based on the nature of polar head groups they are
classified as
Anionic surfactants
Cationic surfactants
Non ionic surfactants
Zwitter ionic surfactants](https://image.slidesharecdn.com/surfactntschristy-170826170814/85/Surfactnts-3-320.jpg)




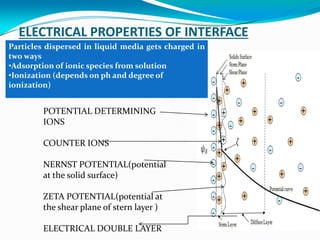
![MICELLE STRUCTURE [ionic, zwitter ionic ]
•Liquid core-
formed by the n
number
hydrocarbon chains
•Stern layer- ionic
head groups + (1-
alpha) n counter
ions
•Diffusion layer or
gouy Chapman
layer- alpha n
counter ions
Location of
interface is
0.08nm above the
alpha carbon of
alkyl chains](https://image.slidesharecdn.com/surfactntschristy-170826170814/85/Surfactnts-11-320.jpg)

![MICELLE STRUCTURE[non ionic]
Typical example of
non ionic micelles is
those formed by
polyoxyethylated
surfactants. Its main
components are
Core
Palisade layer
Core is surrounded
by a layer of
polyoxyethylene
chains to which
solvent molecules
may be hydrogen
bonded. This region
of micelle is called
palisade layer.](https://image.slidesharecdn.com/surfactntschristy-170826170814/85/Surfactnts-13-320.jpg)