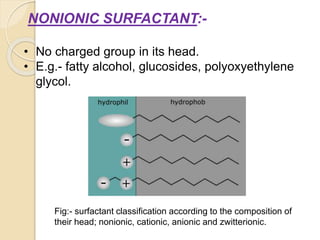
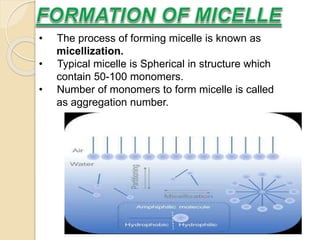
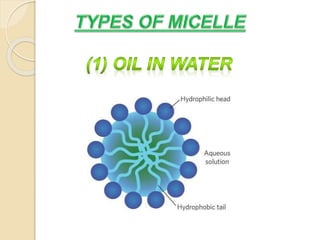
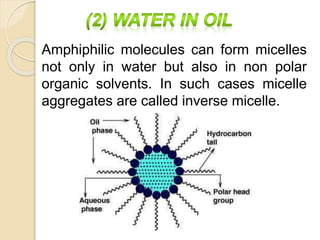
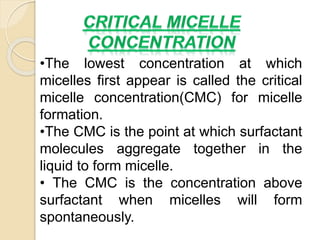
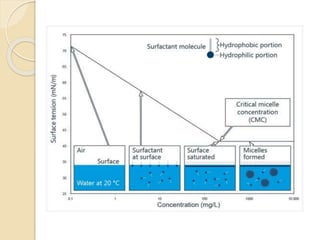
This document discusses surfactants and micelle formation. It defines surfactants as compounds that lower surface tension between liquids and between liquids and solids. Surfactants are amphiphilic, with a hydrophilic head and hydrophobic tail. Micelles are spherical aggregates of surfactant molecules that form in aqueous solutions above a critical concentration known as the critical micelle concentration (CMC). The document classifies surfactants as ionic, nonionic, cationic, anionic or zwitterionic and discusses their structures. It also outlines common uses of surfactants and types of micelles that can form.