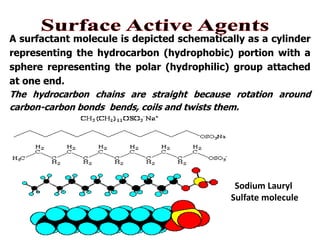
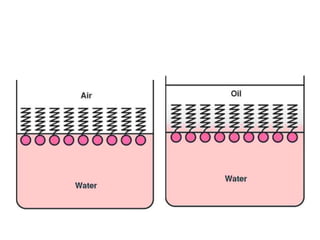
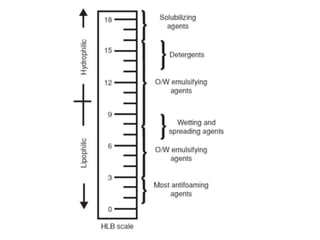
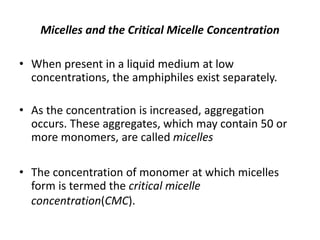
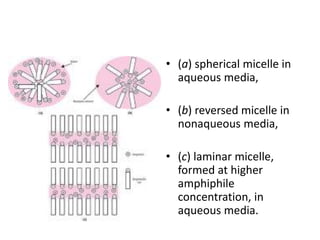
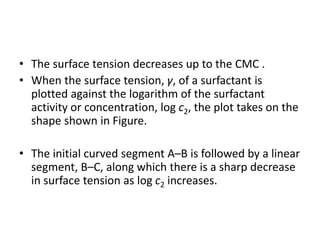
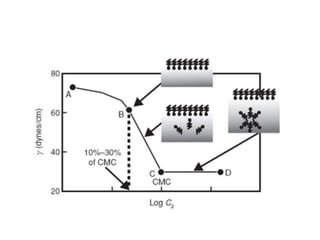
This document discusses surfactants and their properties including critical micelle concentration and hydrophilic lipophilic balance. It describes how surfactant molecules have both hydrophilic and hydrophobic portions which allow them to accumulate at interfaces. It explains that surfactants exist separately at low concentrations but aggregate into micelles above the critical micelle concentration. The surface tension decreases with increasing surfactant concentration up until the CMC. Micelle formation and solubilization are discussed. The document also briefly mentions solid-gas and solid-liquid adsorption applications of surfactants.