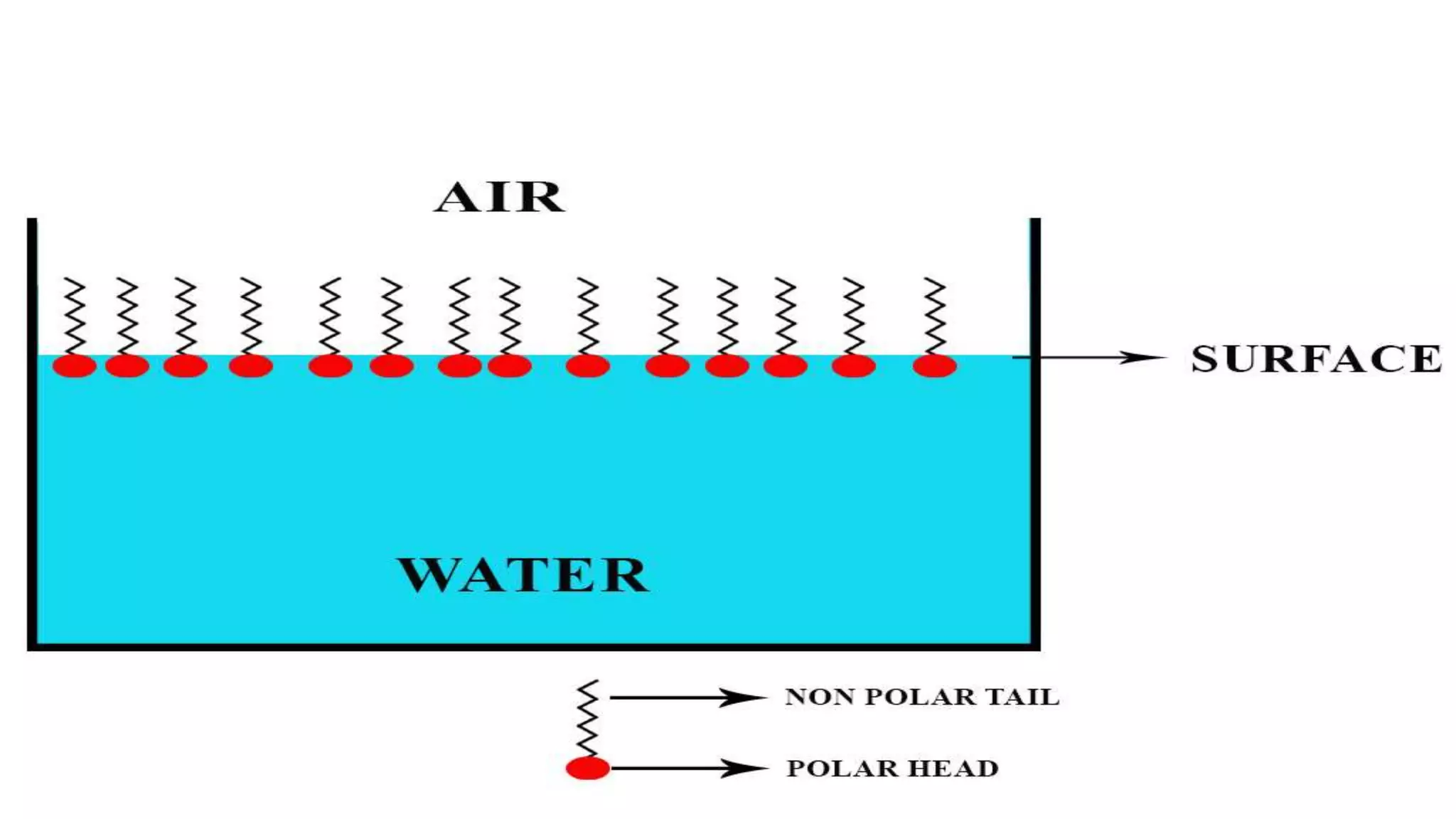
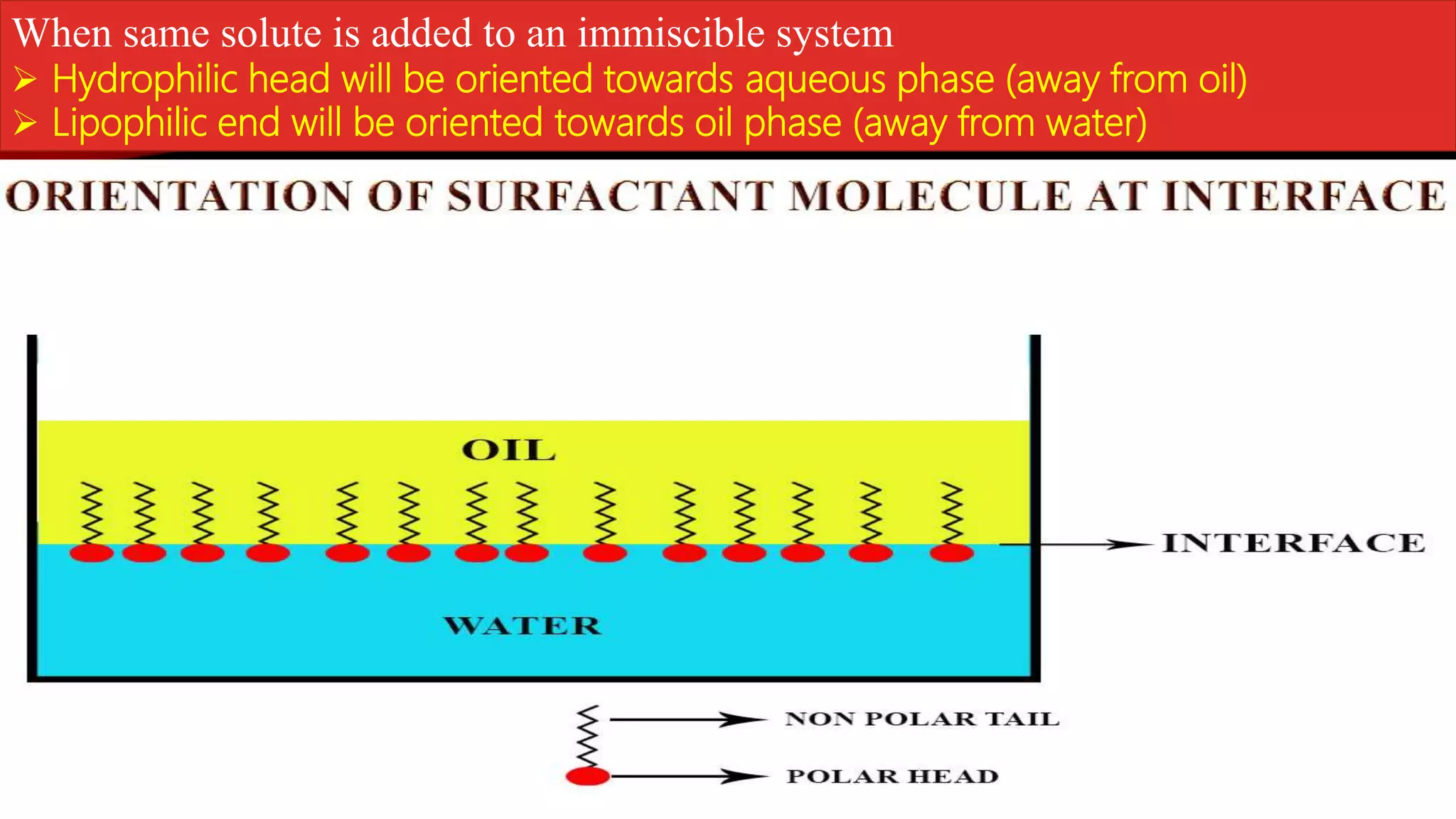
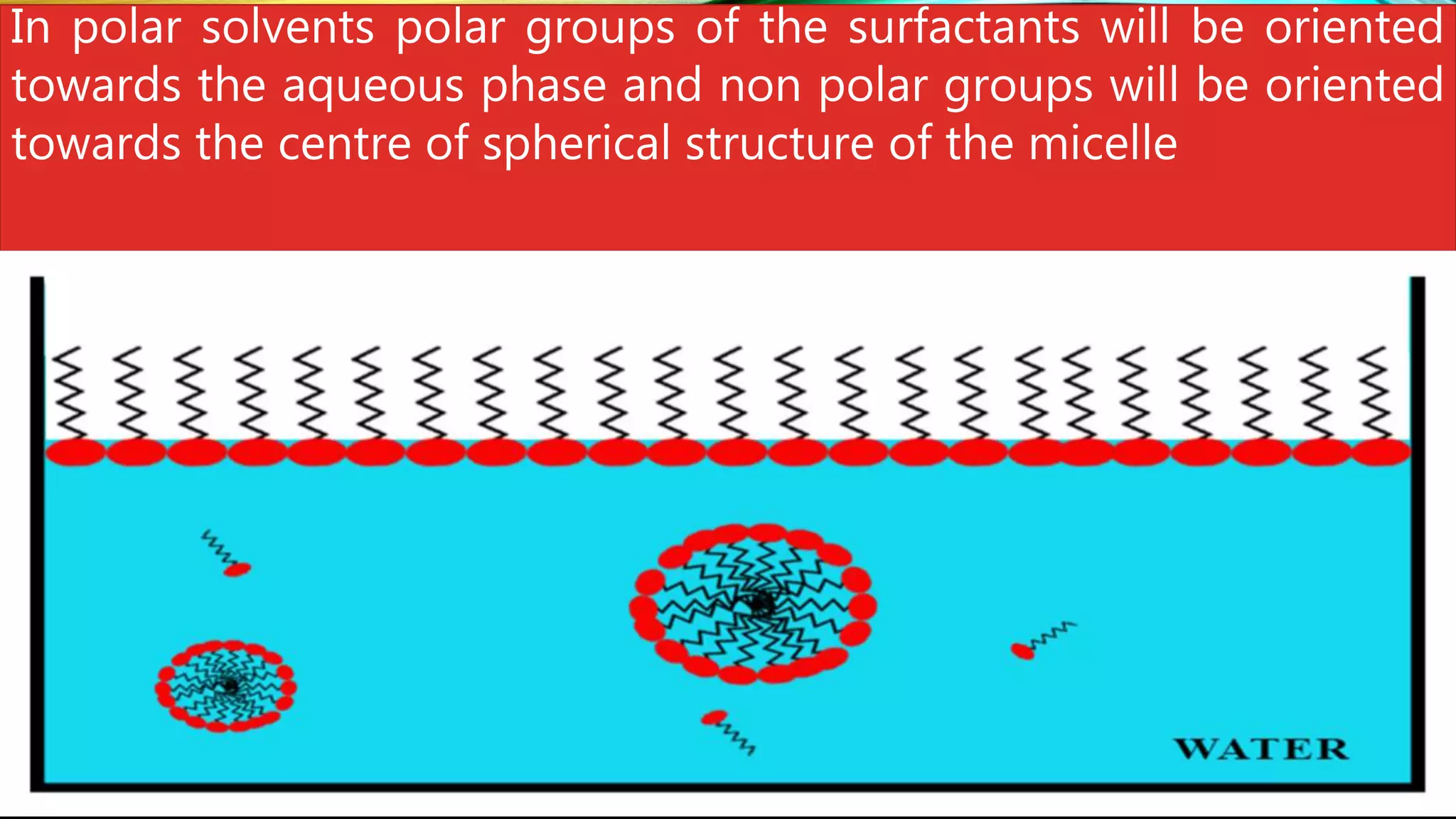
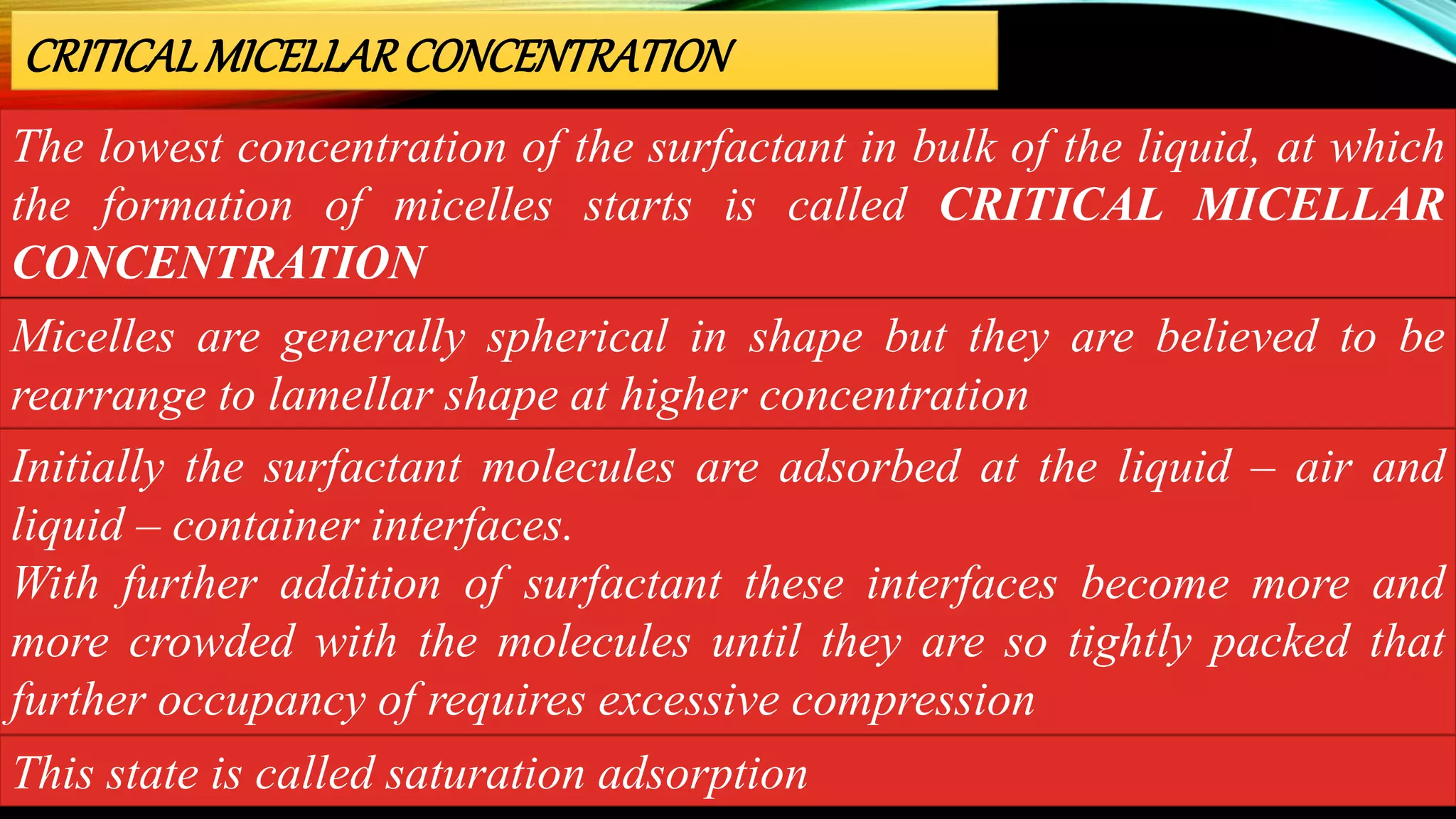
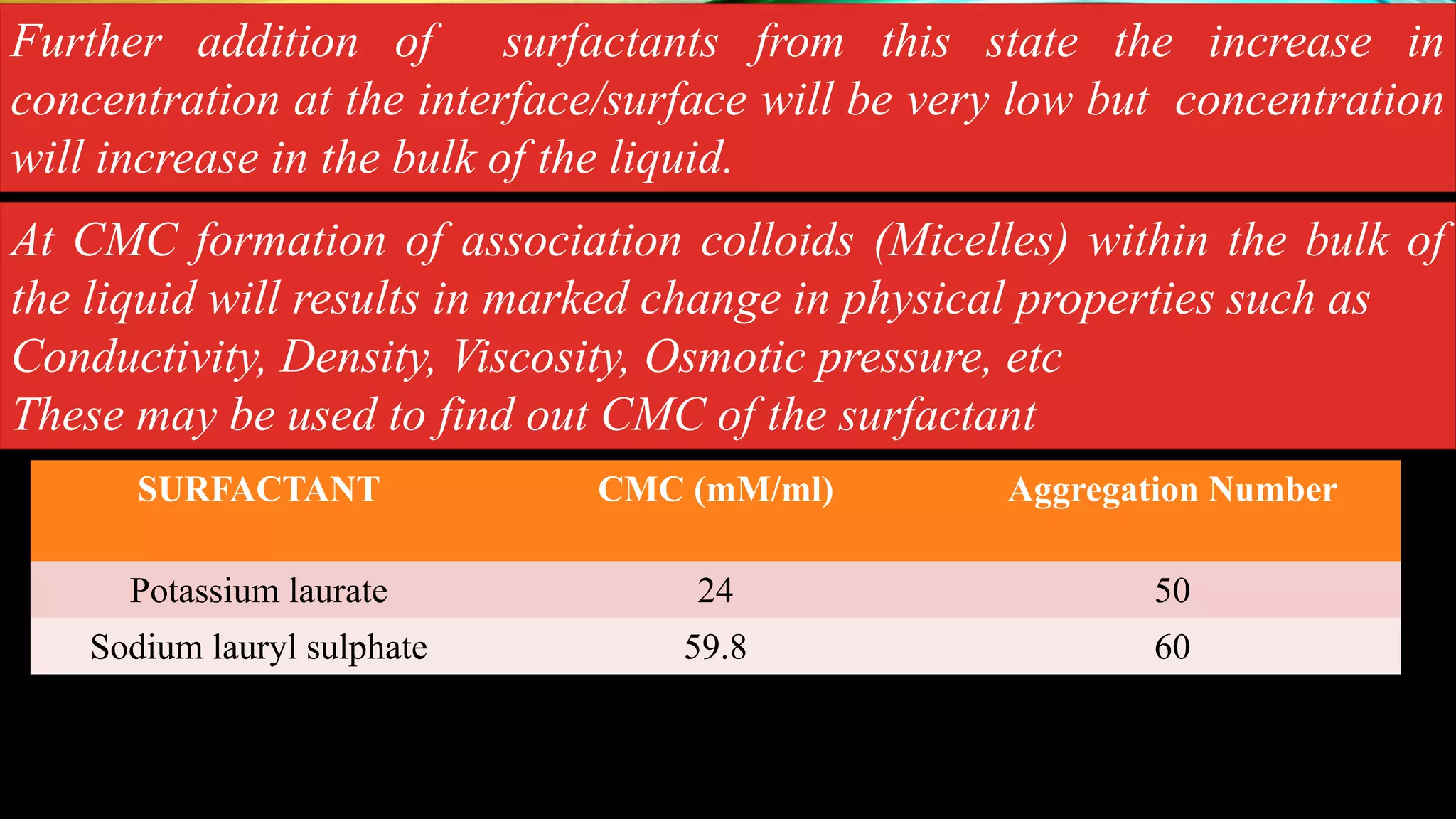
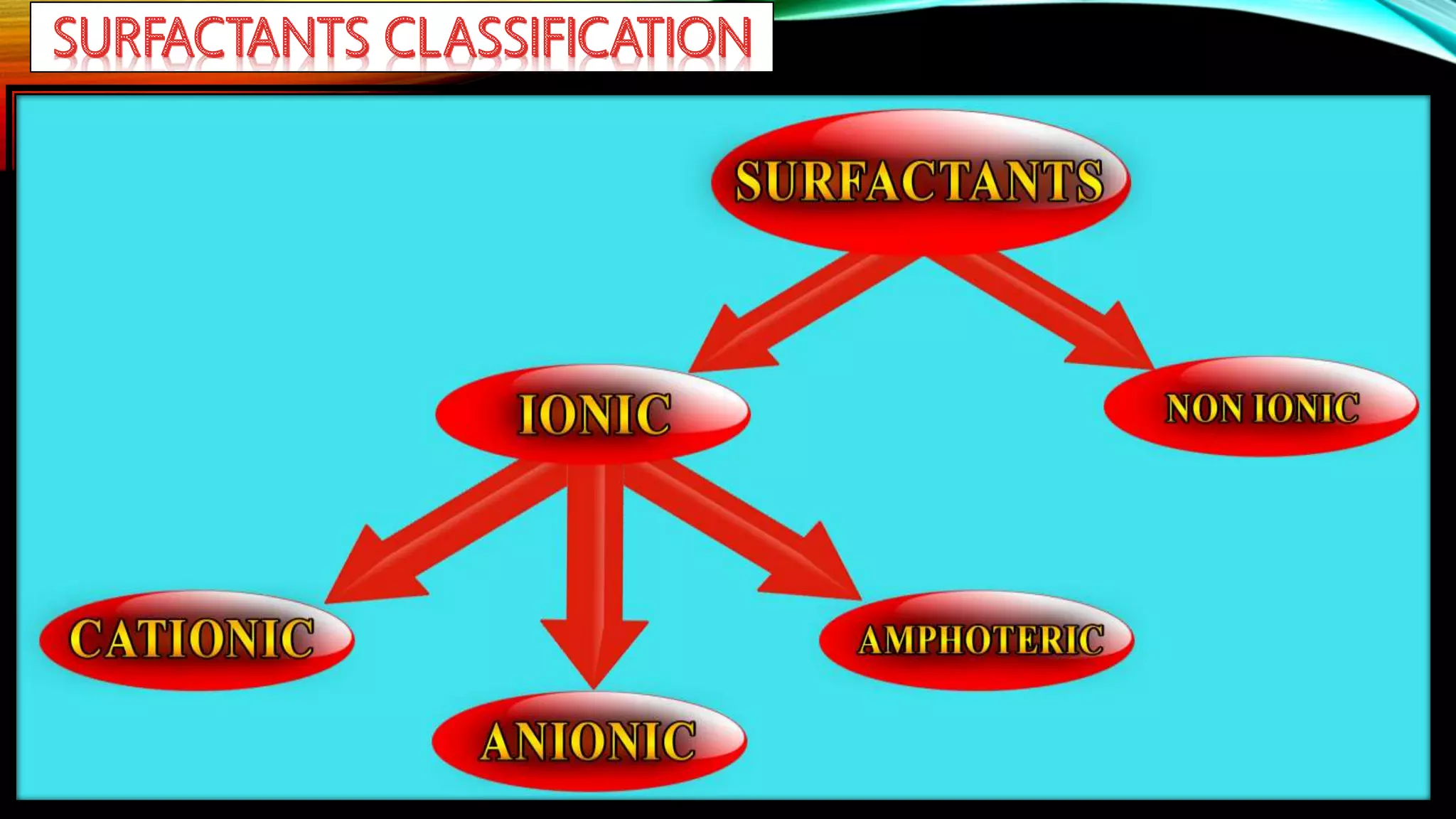
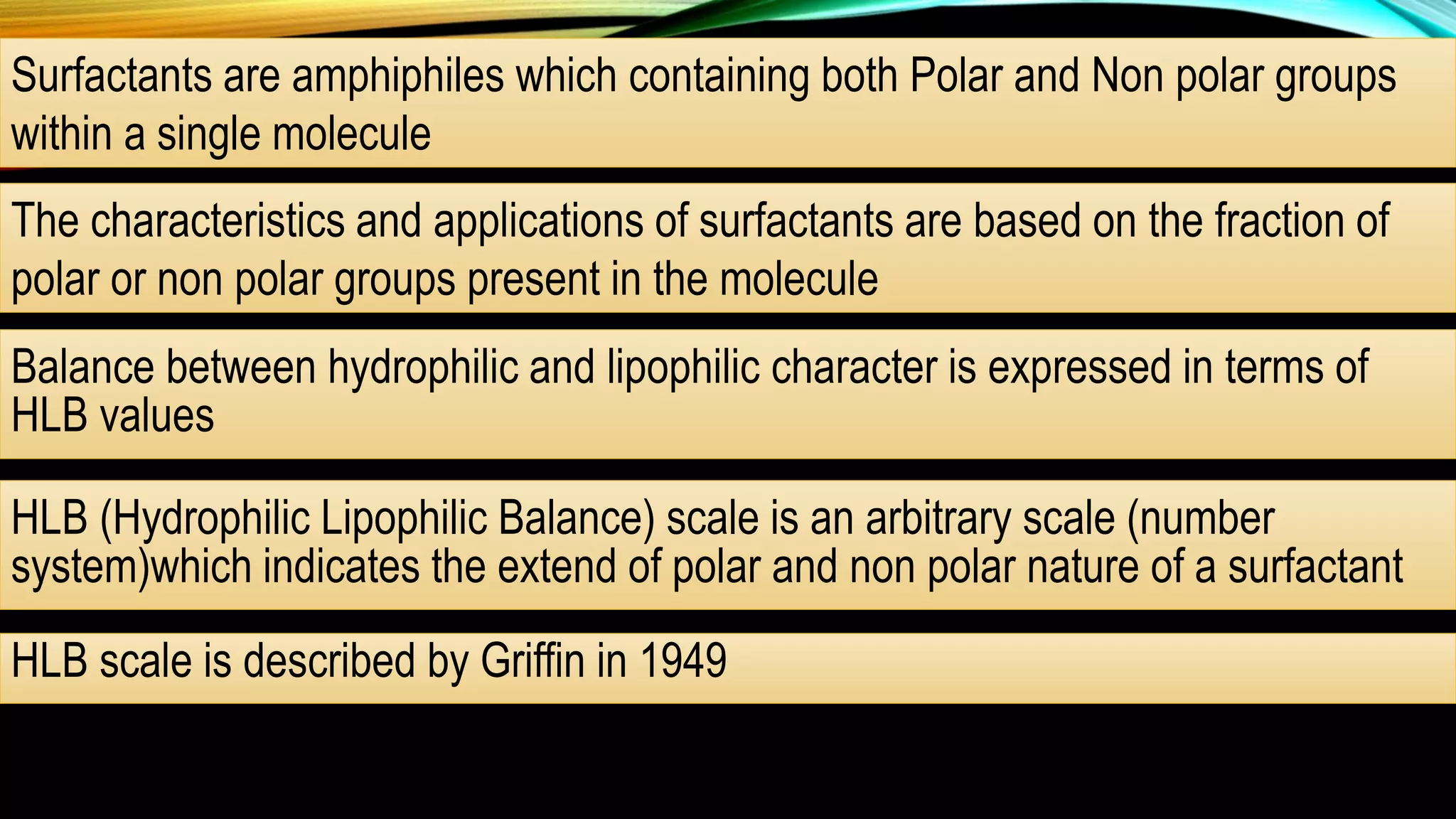
This document discusses adsorption at liquid surfaces and interfaces. It defines positive and negative adsorption, where some solute molecules are partitioned in favor of the surface/interface (positive adsorption) while others favor the bulk liquid (negative adsorption). Surfactant molecules that reduce surface/interfacial tension through positive adsorption are also introduced. The document then describes how surfactant molecules have both a polar head and nonpolar tail, and how they orient at interfaces. It discusses micelle formation above the critical micelle concentration and how this affects physical properties. In summary, the document provides an overview of adsorption phenomena at liquid surfaces/interfaces with a focus on surfactant molecules and micelle formation.