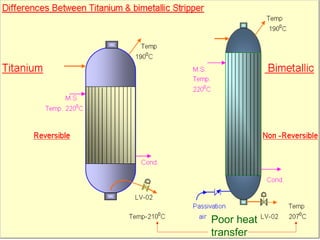
This document provides an overview of materials used in fertilizer plants, including their classification, properties, and applications. It discusses various types of metals and alloys used, including carbon steel, cast iron, stainless steel, and others. Key points covered include:
- Classification of materials into ferrous, non-ferrous, metallic, and non-metallic categories.
- Properties of materials like strength, hardness, ductility, and toughness.
- Types of steel alloys and role of elements like chromium, nickel, molybdenum, and carbon.
- Applications of materials for cooling water networks, steam lines, and urea service equipment.
- Stainless steel