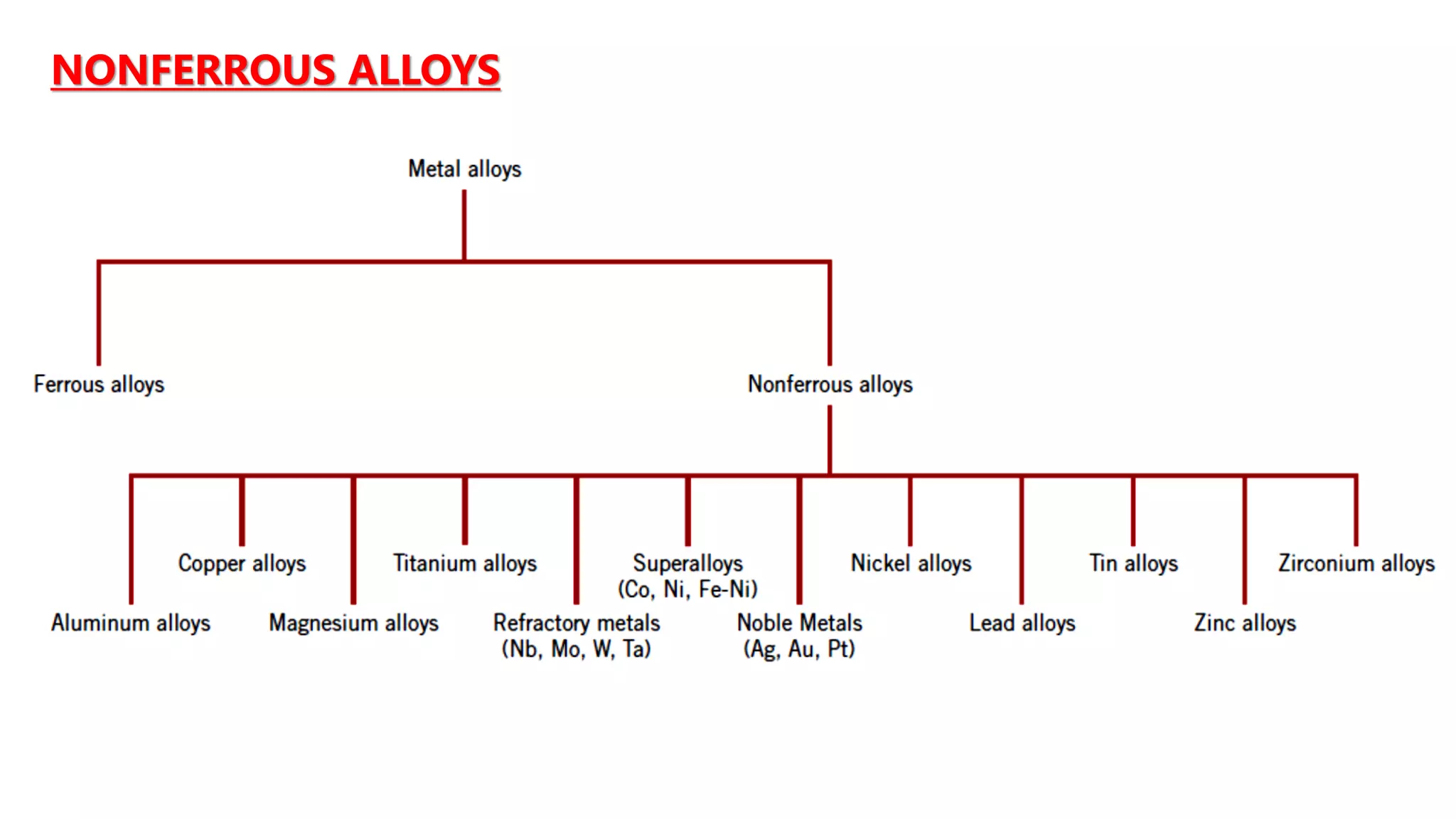
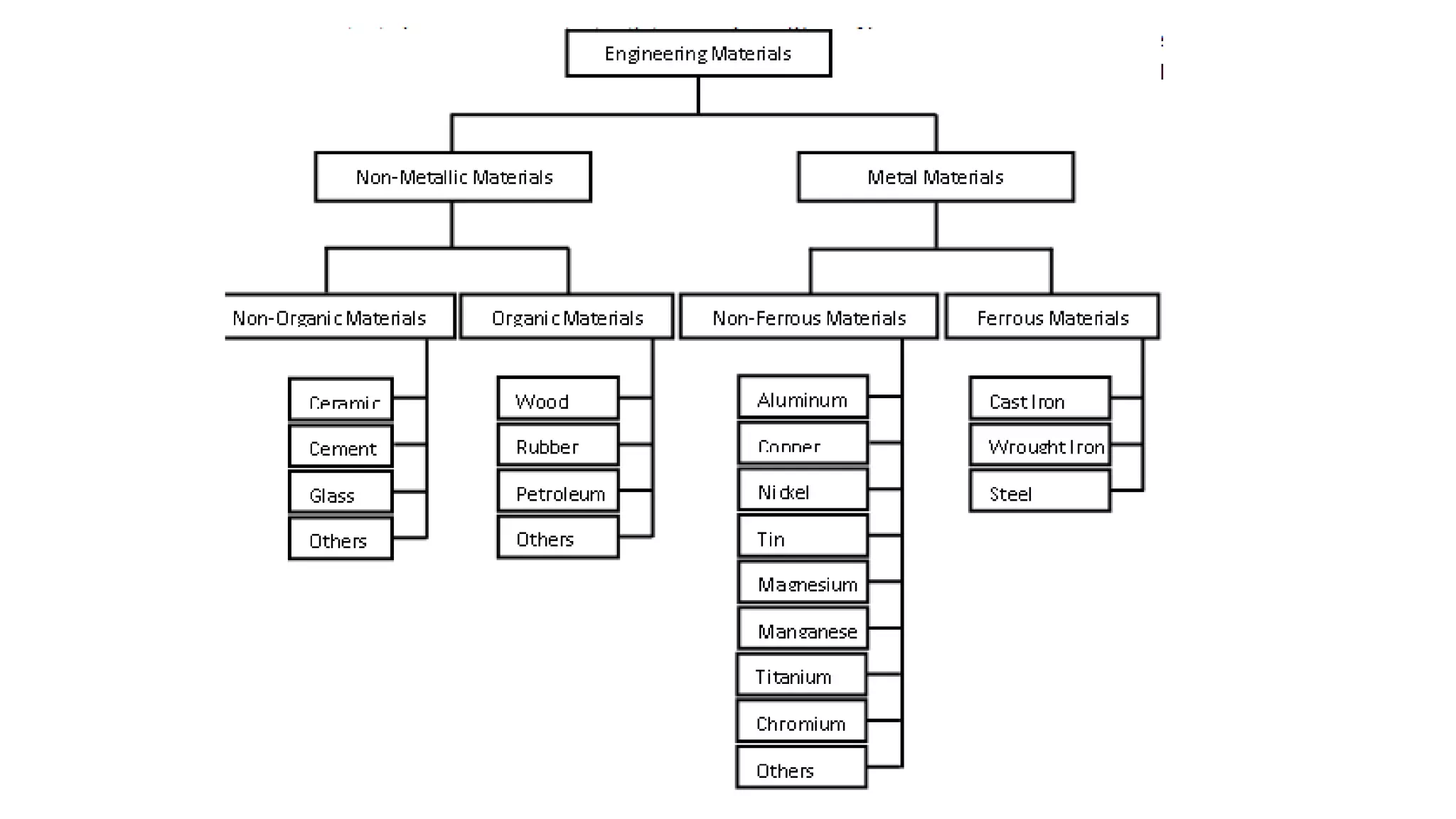
1. Carbon steels are iron alloys with varying amounts of carbon. Low-carbon steel is ductile while high-carbon steel is strong.
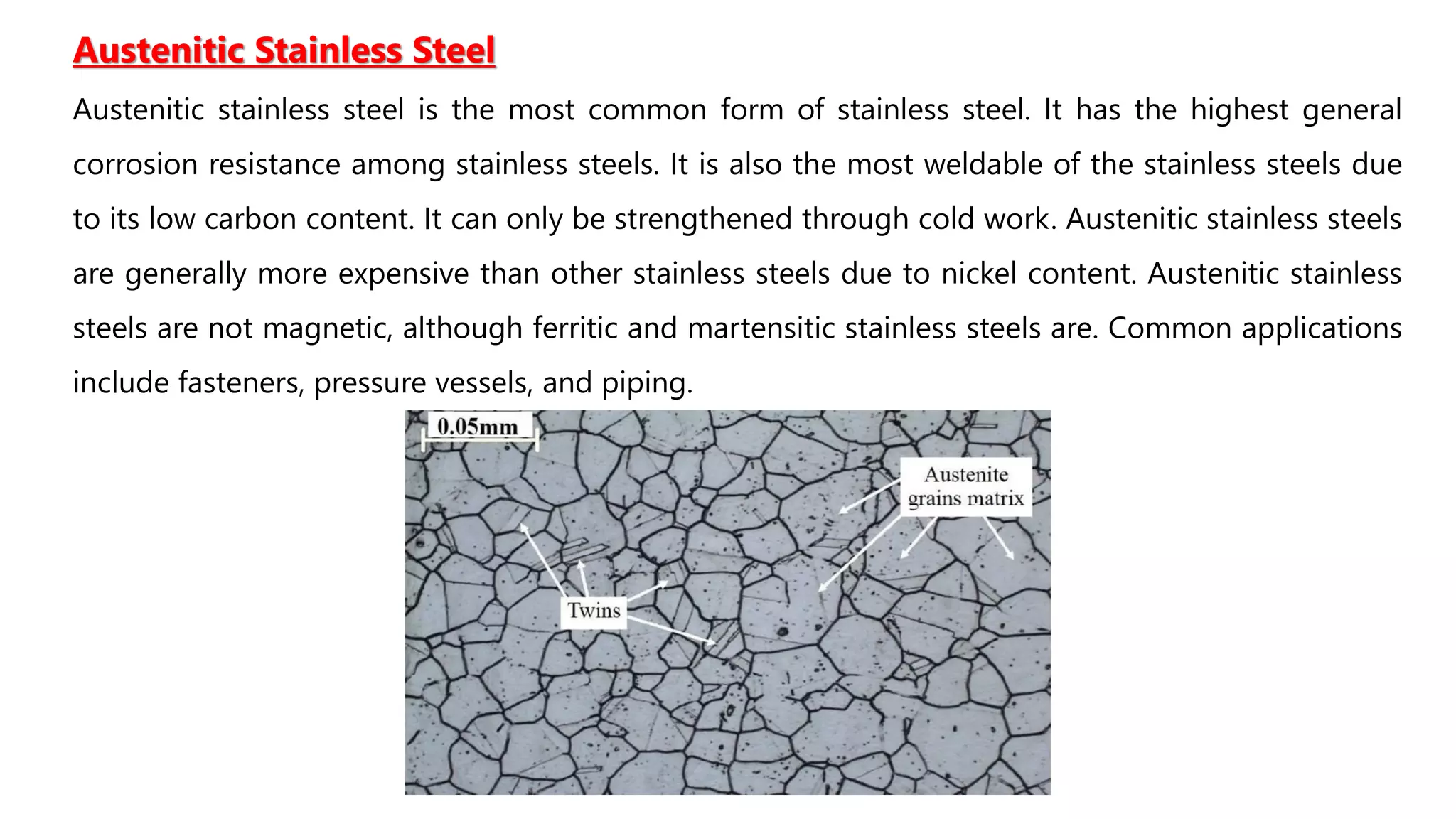
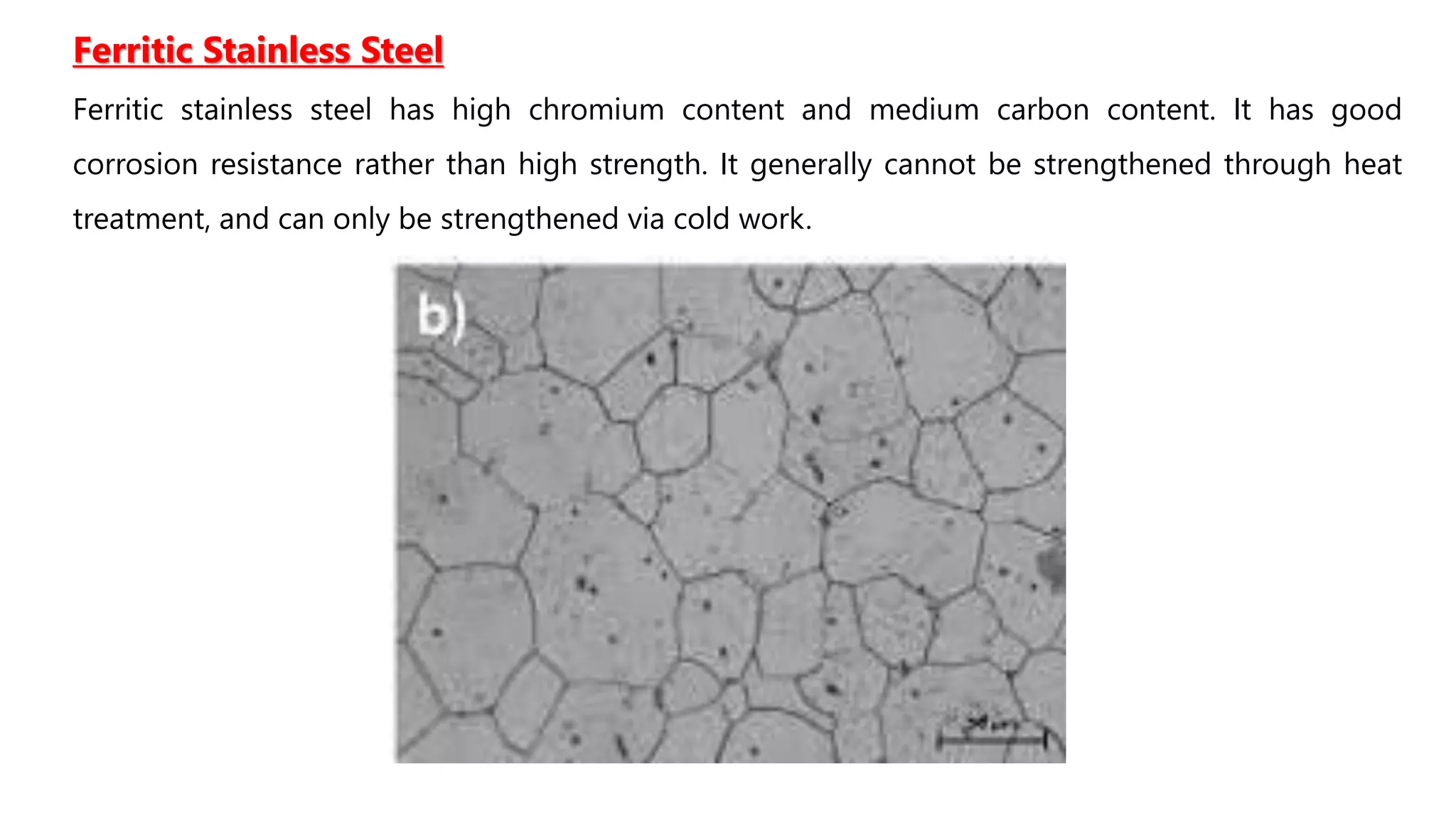
2. Stainless steels contain at least 11% chromium which allows a protective oxide layer to form, increasing corrosion resistance. Common types are austenitic, ferritic, and martensitic stainless steels.
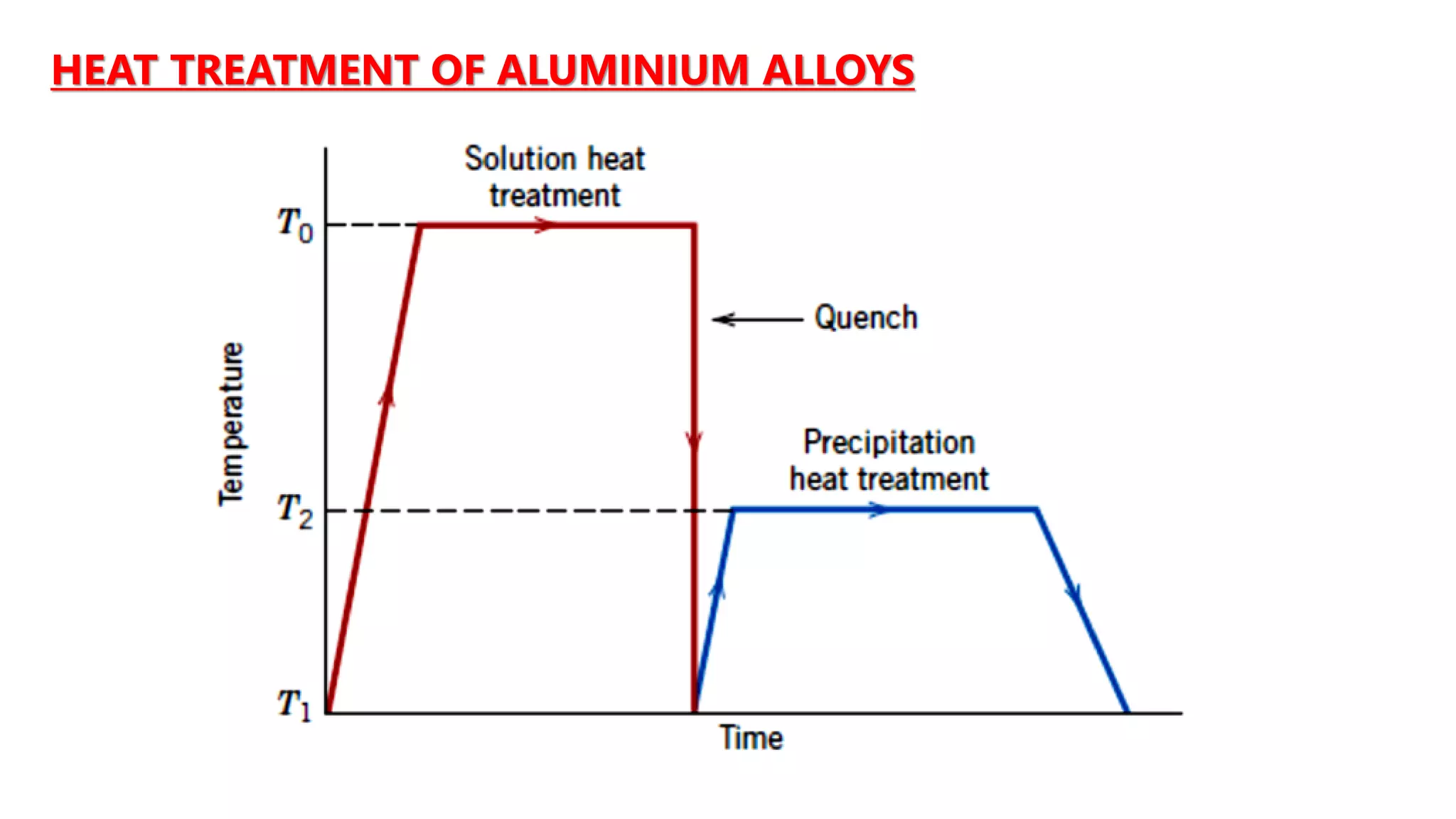
3. Heat treatments can be used to alter the properties of cast irons and steels. Annealing softens materials while hardening increases strength and wear resistance. Cryogenic treatment further enhances properties.




















![Spheroidizing
Medium- and high-carbon steels having a microstructure containing even coarse pearlite may
still be too hard to machine or plastically deform conveniently. These steels, and in fact any
steel, may be heat-treated or annealed to develop the spheroidite structure. Spheroidized
steels have a maximum softness and ductility and are easily machined or deformed. The
spheroidizing heat treatment, during which there is a coalescence of the Fe3C to form the
spheroid particles, can take place by several methods, as follows:
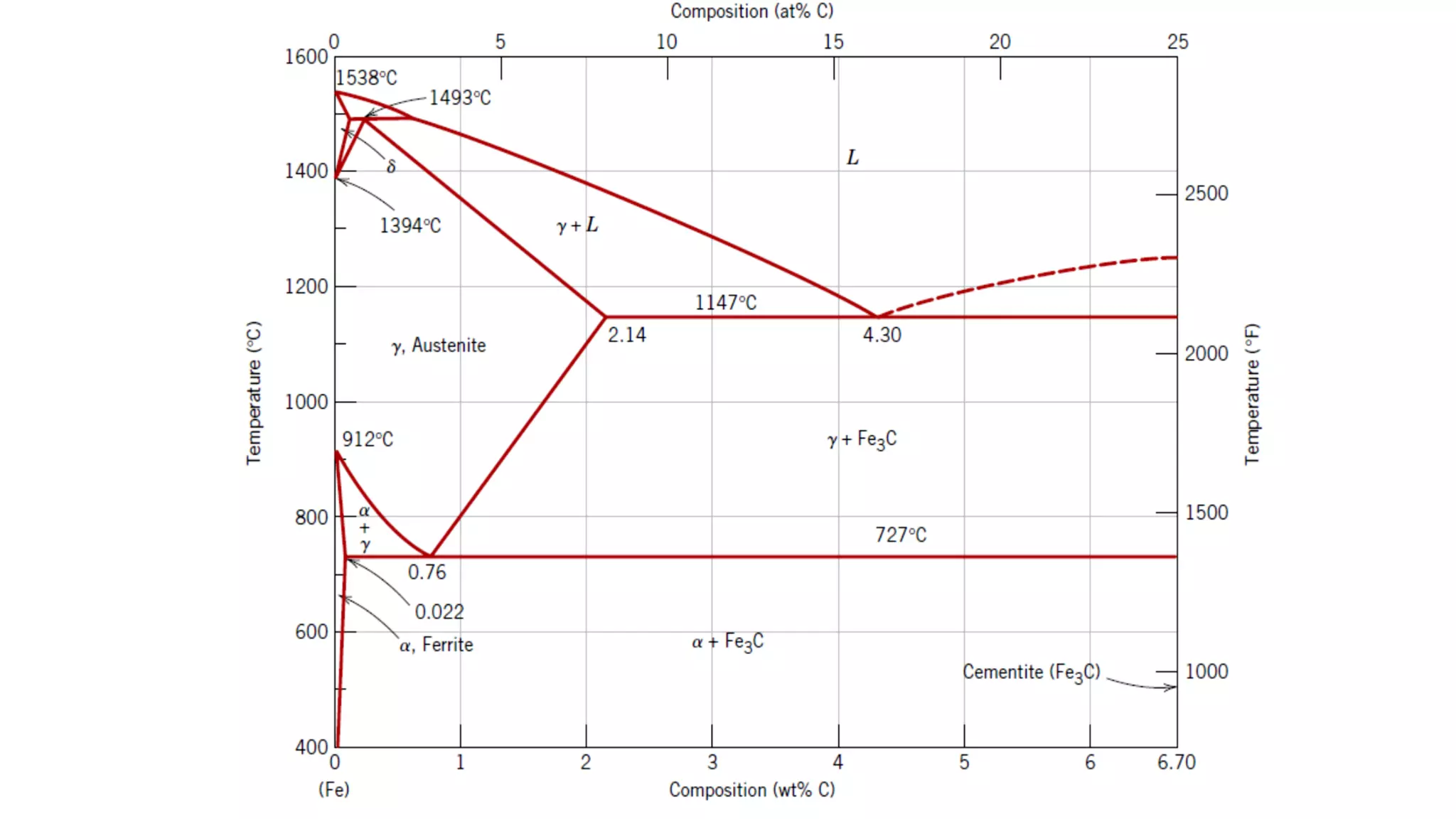
• Heating the alloy at a temperature just below the eutectoid [line A1 in Figure 31, or at about
700ºC (1300ºF)] in the α+Fe3C region of the phase diagram. If the precursor microstructure
contains pearlite, spheroidizing times will typically range between 15 and 25 h.
• Heating to a temperature just above the eutectoid temperature and then either cooling very
slowly in the furnace or holding at a temperature just below the eutectoid temperature.
• Heating and cooling alternately within about ± 50ºC of the A1 line.](https://image.slidesharecdn.com/amm-unit1-230808102930-a789a789/75/AMM-UNIT-1-pdf-27-2048.jpg)