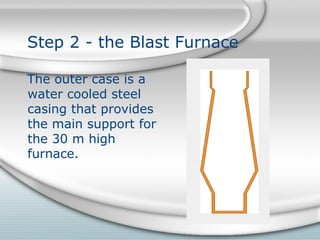
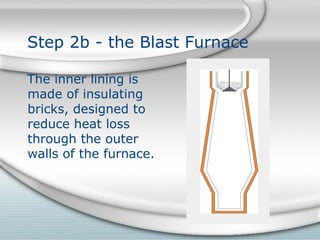
The document describes the process of forming iron and steel using a blast furnace. It involves the following key steps:
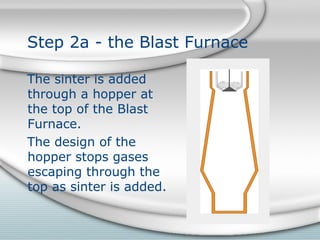
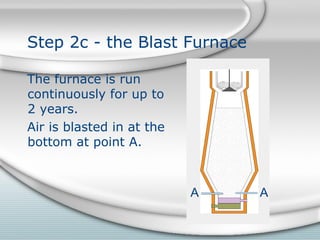
1. Sinter is added to the top of the blast furnace. Air is blasted into the bottom to fuel reactions that melt the iron out of the sinter.
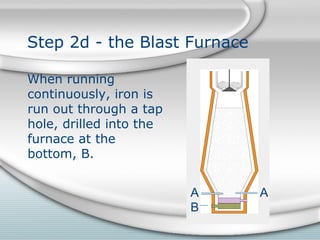
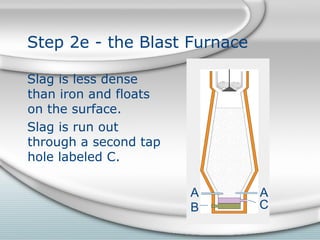
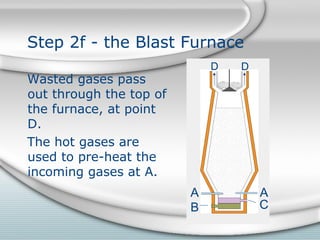
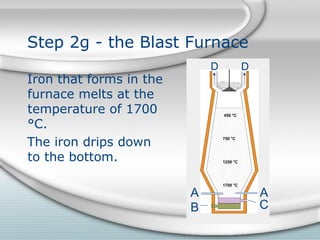
2. Molten iron collects at the bottom of the furnace and is tapped out periodically. Slag floats on top and is also tapped out. Wasted gases exit from the top.
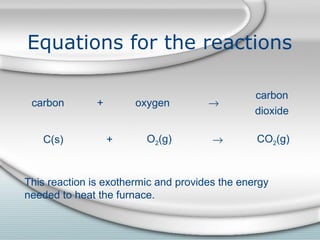
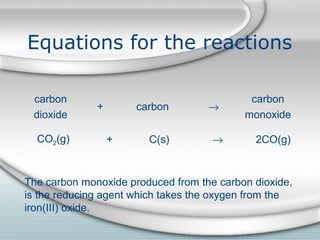
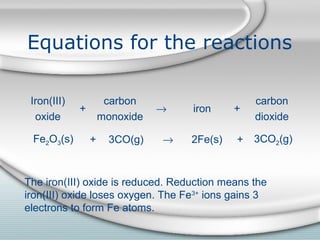
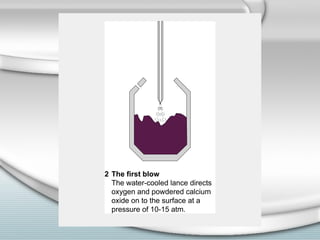
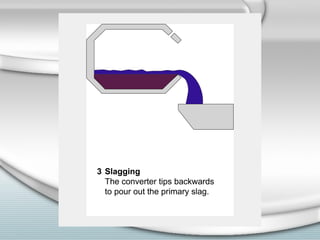
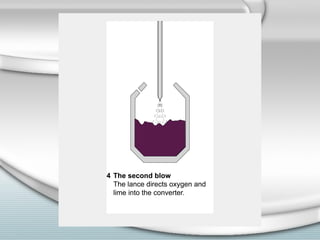
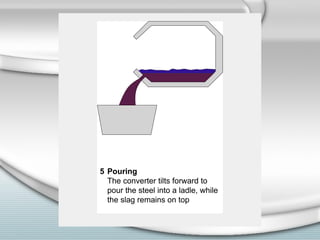
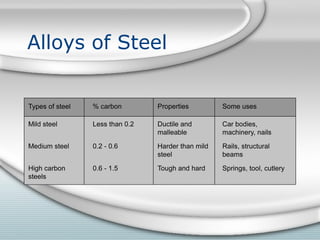
3. The iron produced contains carbon and impurities, making it cast iron. Steel is made by removing carbon from cast iron through oxidation, then adding other metals to produce alloys with specific properties.