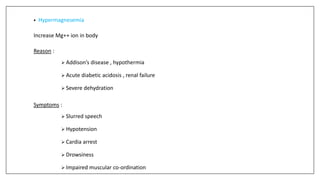
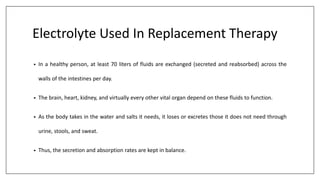
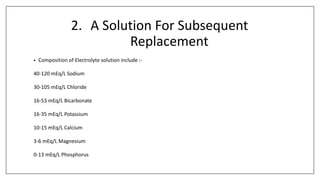
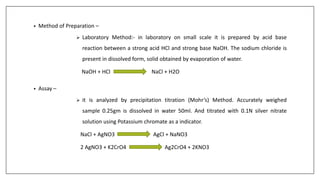
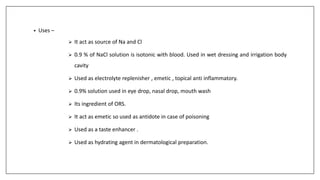
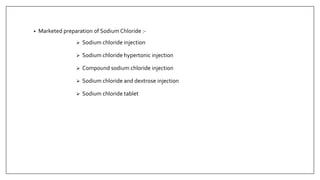
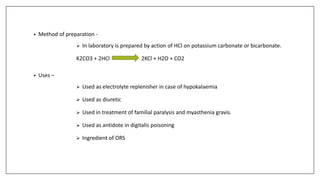
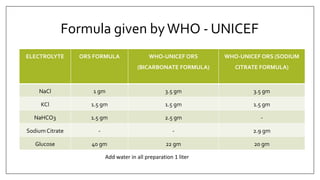
The document discusses the types of body fluids and the role of major electrolytes such as sodium, calcium, chloride, potassium, and magnesium in maintaining homeostasis and physiological functions. It details the causes, symptoms, and treatments for imbalances like hyponatremia, hyperkalemia, and hypocalcemia, along with their dietary sources and replacement therapies. Additionally, it covers the importance of electrolytes in acid-base balance and various methods of electrolyte replacement therapy in clinical settings.


















![Physiological Acid-Base Balance
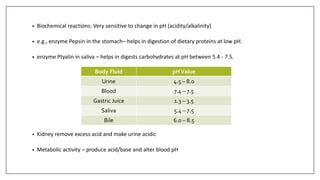
• Electrolytes also play an important role in regulating body’s acid-base balance
• Body fluids contain balanced quantities of acids & bases.
• Acidity of the solution: No of [H+ ] present in fluid/solution - ECF
• Sources: [H+ ]
• Food
• Cellular metabolism of Glucose, Fatty acids, & Amino acids etc
• Reabsorption](https://image.slidesharecdn.com/12-210315055144/85/MAJOR-INTRA-EXTRA-CELLULAR-ELECTROLYTE-33-320.jpg)