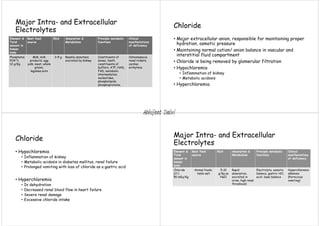
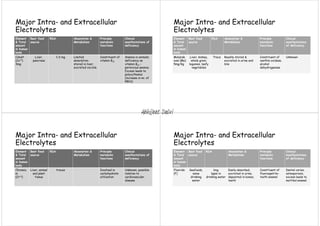
Major extracellular and intracellular electrolytes maintain homeostasis through regulatory mechanisms that control pH, ionic balances, and osmotic balances. The three fluid compartments - intracellular fluid, interstitial fluid, and plasma - contain varying amounts of major electrolytes including sodium, potassium, chloride, bicarbonate, calcium, magnesium, and phosphate. Imbalances in electrolyte levels can disrupt cellular functions and cause conditions like hypokalemia, hypernatremia, or hypocalcemia. Precise control of electrolyte concentrations is vital for normal physiological activities.