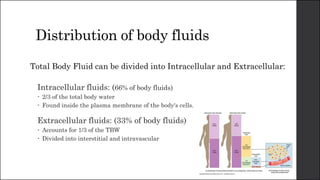
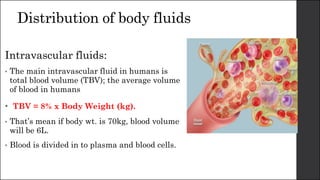
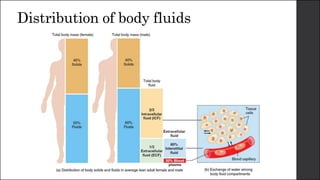
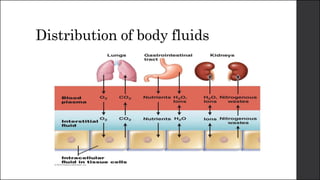
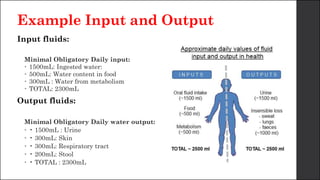
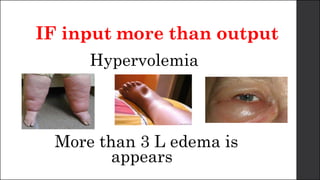
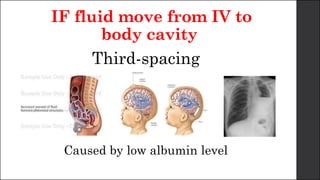
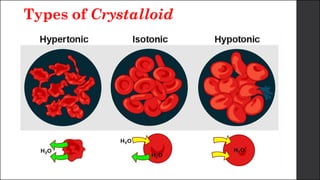
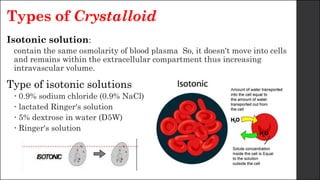
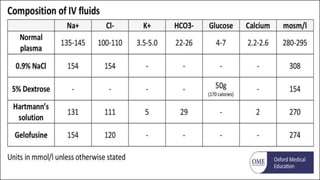
The document discusses body fluid distribution and components, explaining that total body fluid constitutes about 60% of body weight and can be divided into intracellular and extracellular fluids. It outlines different types of solutions used in medical contexts, such as isotonic, hypotonic, and hypertonic solutions, along with their uses and precautions. Additionally, it highlights the importance of fluid volume assessment and monitoring in maintaining normal body function.