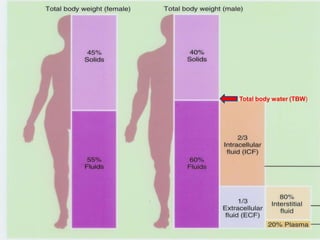
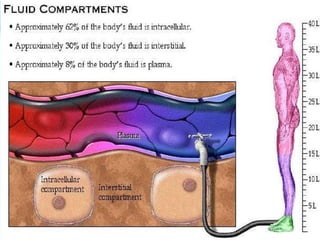
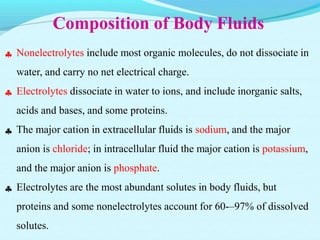
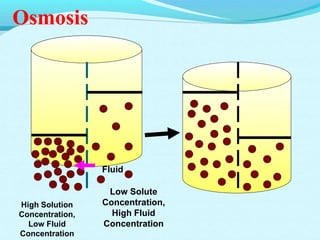
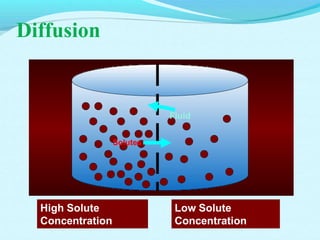
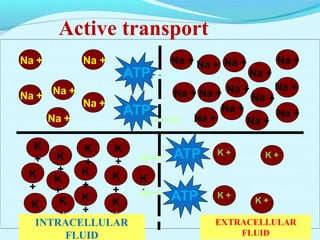
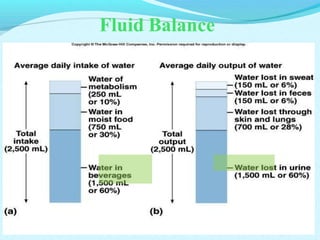
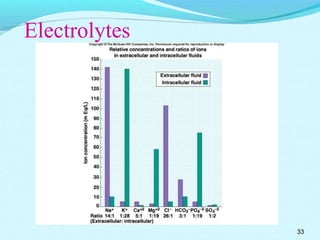
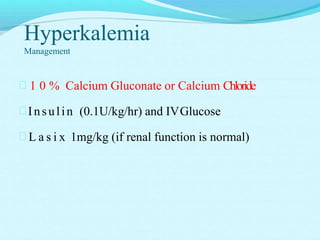
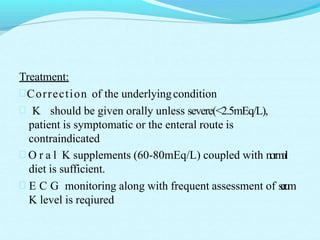
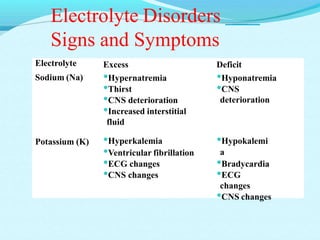
The document provides an extensive overview of body fluids, their sources, functions, composition, and balance, crucial for maintaining homeostasis. It details fluid movements, electrolyte balance, and the effects of various disorders, including hypernatremia and hypokalemia. It also discusses the physiological regulation of water and electrolyte levels, factors affecting fluid balance, and recommendations for managing electrolyte abnormalities.