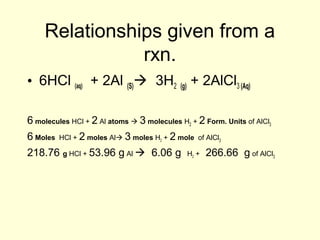
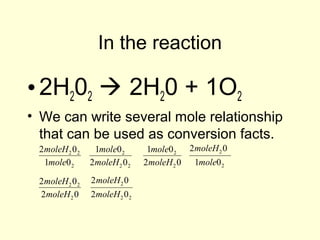
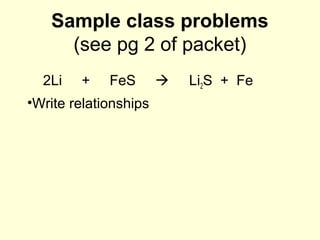
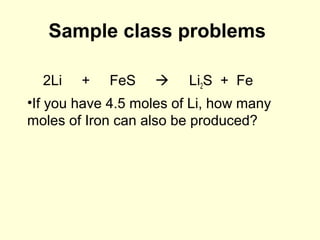
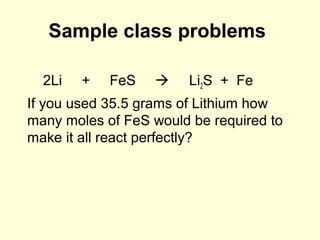
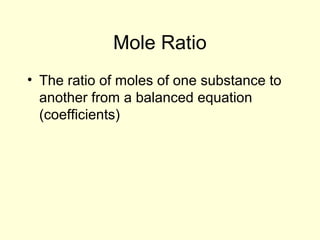
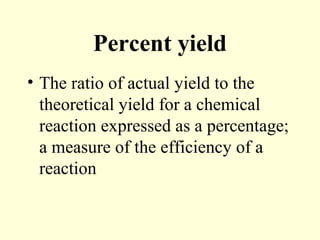
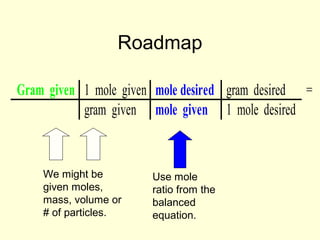
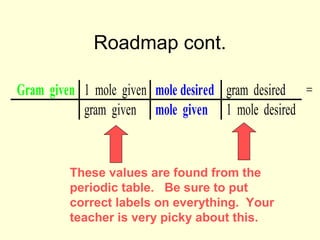
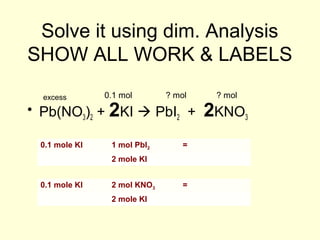
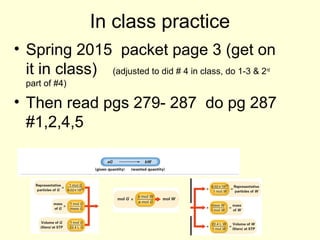
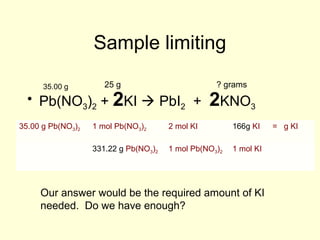
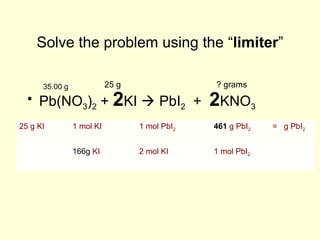
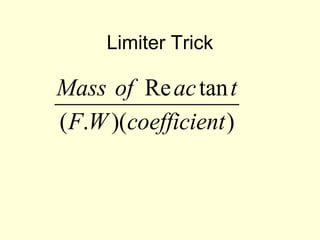
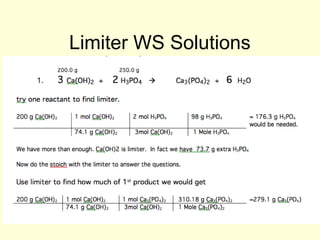
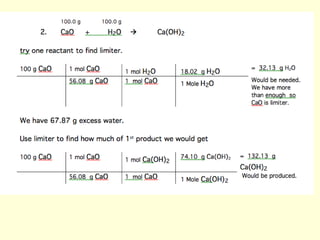
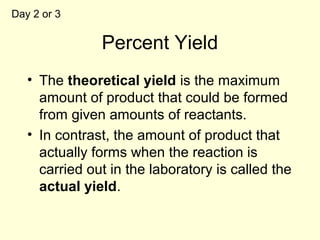
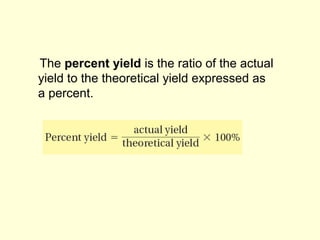
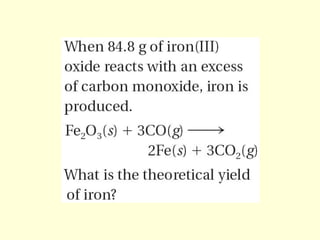
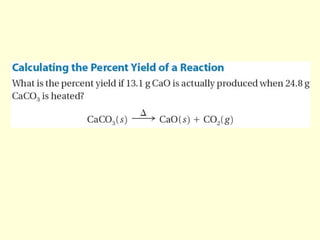
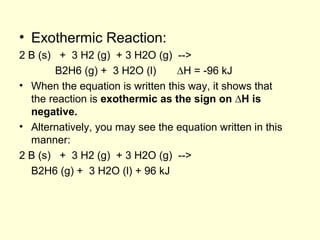
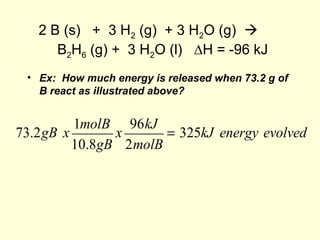This document provides information about stoichiometry concepts including mole ratios, limiting reagents, theoretical and actual yields, and percent yields. It gives examples of mole-mole, gram-gram, and gram-liter conversions using balanced chemical equations. Heat of reaction is discussed, including signs for exothermic and endothermic reactions. Sample problems are provided for limiting reagents, percent yields, and heat of reaction calculations. Worksheets are assigned to practice these stoichiometry skills in preparation for a test involving mole-mole, mass, volume, heat, yield, and limiting reagent conversions and calculations.