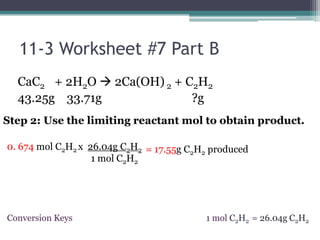
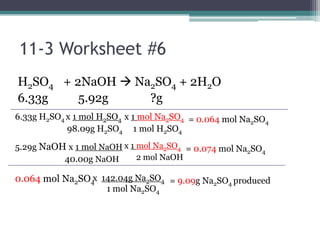
The document discusses reaction stoichiometry and determining limiting and excess reactants. It provides examples to illustrate:
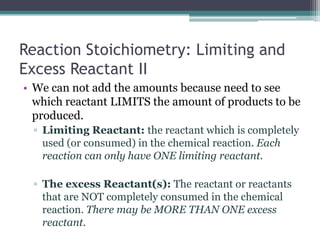
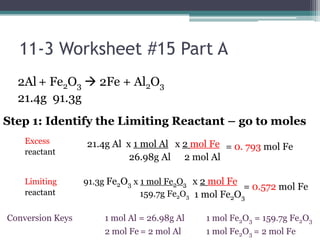
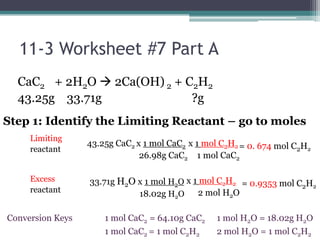
1) When given amounts of multiple reactants, you cannot assume you can simply add the amounts, as one reactant may be limiting.
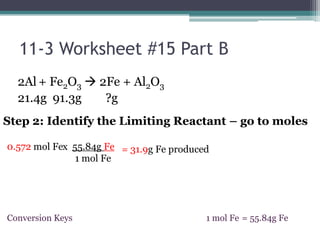
2) The limiting reactant is the one that is completely consumed in the reaction, while excess reactants remain afterward.
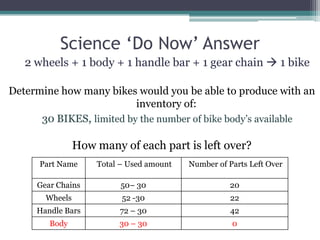
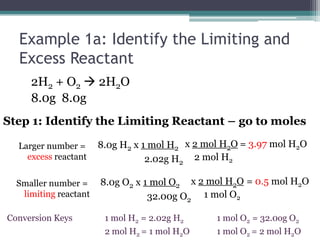
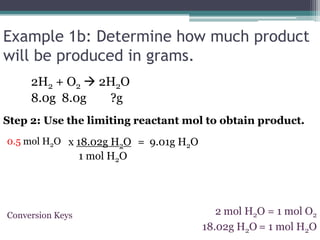
3) Worked examples show how to calculate the limiting reactant by converting masses to moles and identifying which is the smaller amount, and using the limiting reactant to calculate the mass of products formed.