Embed presentation
Downloaded 110 times


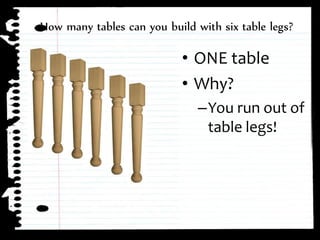
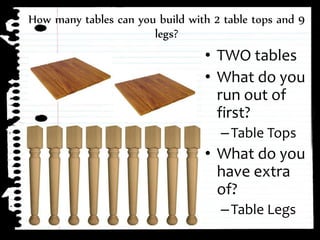




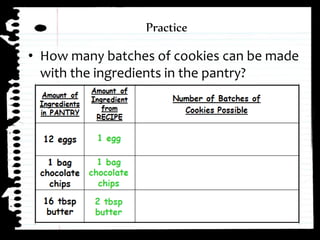
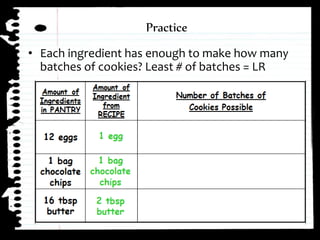



The document defines limiting reactants as the reactant that is used up first in a chemical reaction and limits the amount of product that can be formed. The limiting reactant determines how many reactions can take place and how much of the excess reactant will be left over after the reaction stops due to the limiting reactant running out. Examples are provided to demonstrate identifying the limiting and excess reactants given the amounts of reactants available.











