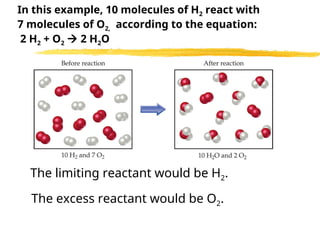
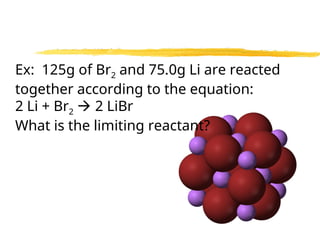
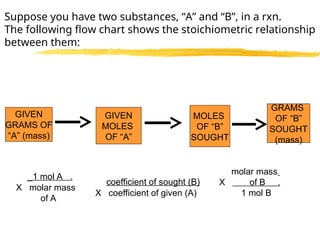
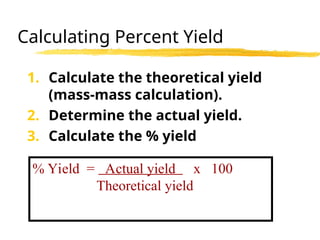
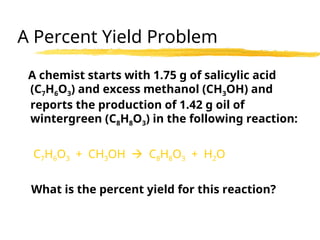
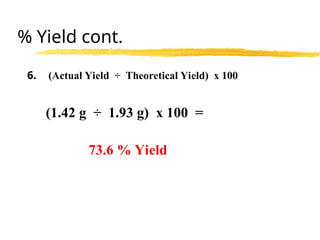
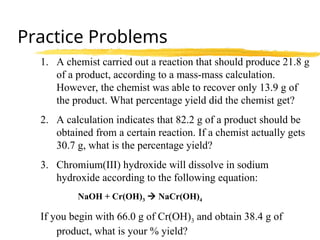
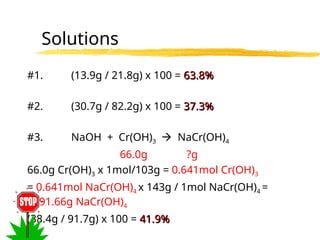
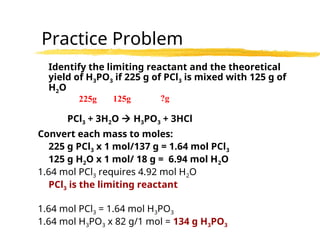
The document explains limiting reactants and percent yield in chemical reactions, providing examples and calculations for determining which reactant limits product formation and how to calculate actual versus theoretical yield. It includes practice problems to help reinforce understanding of these concepts. The strategies for identifying limiting reactants and calculating percent yield are outlined systematically.