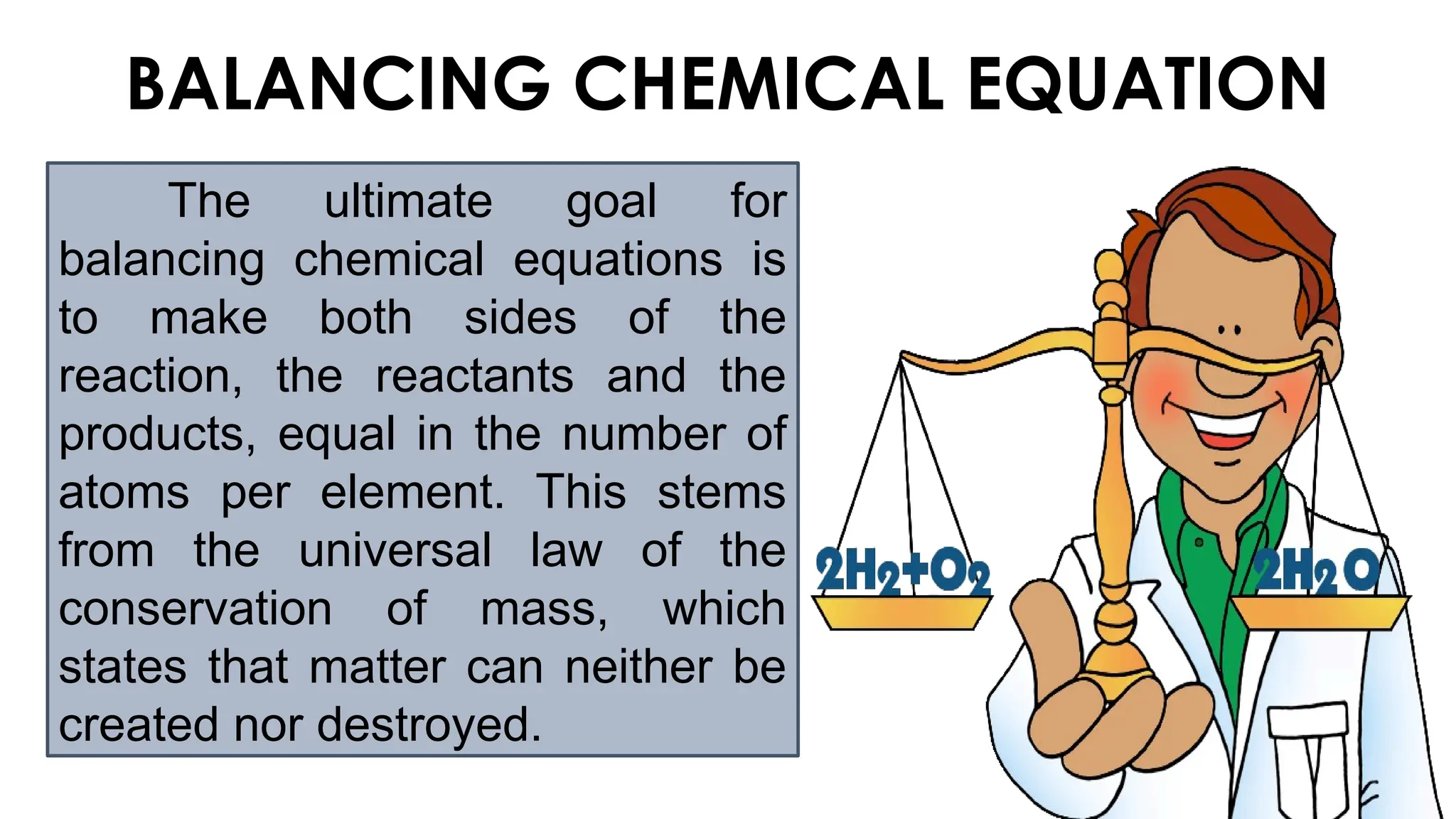
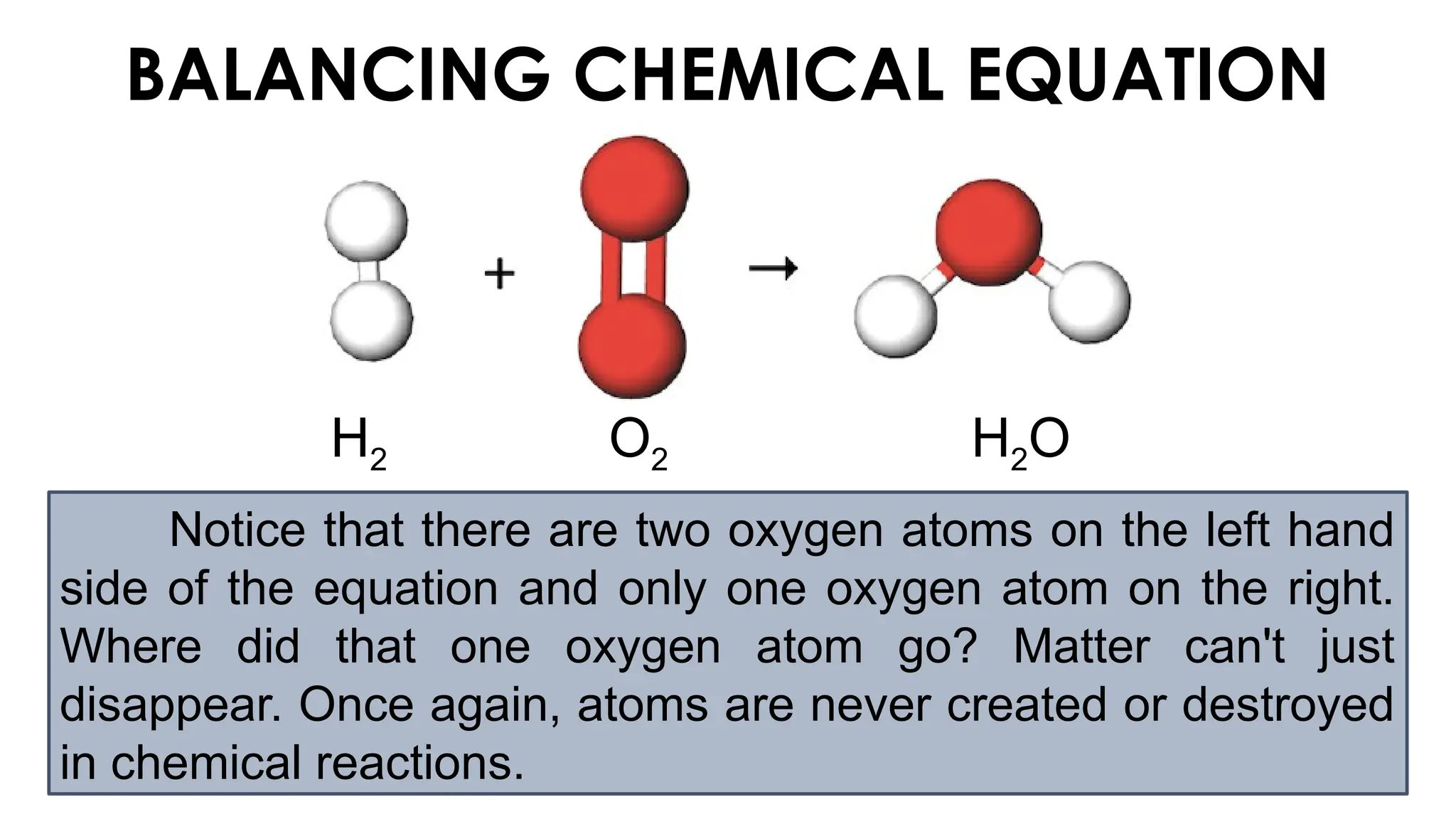
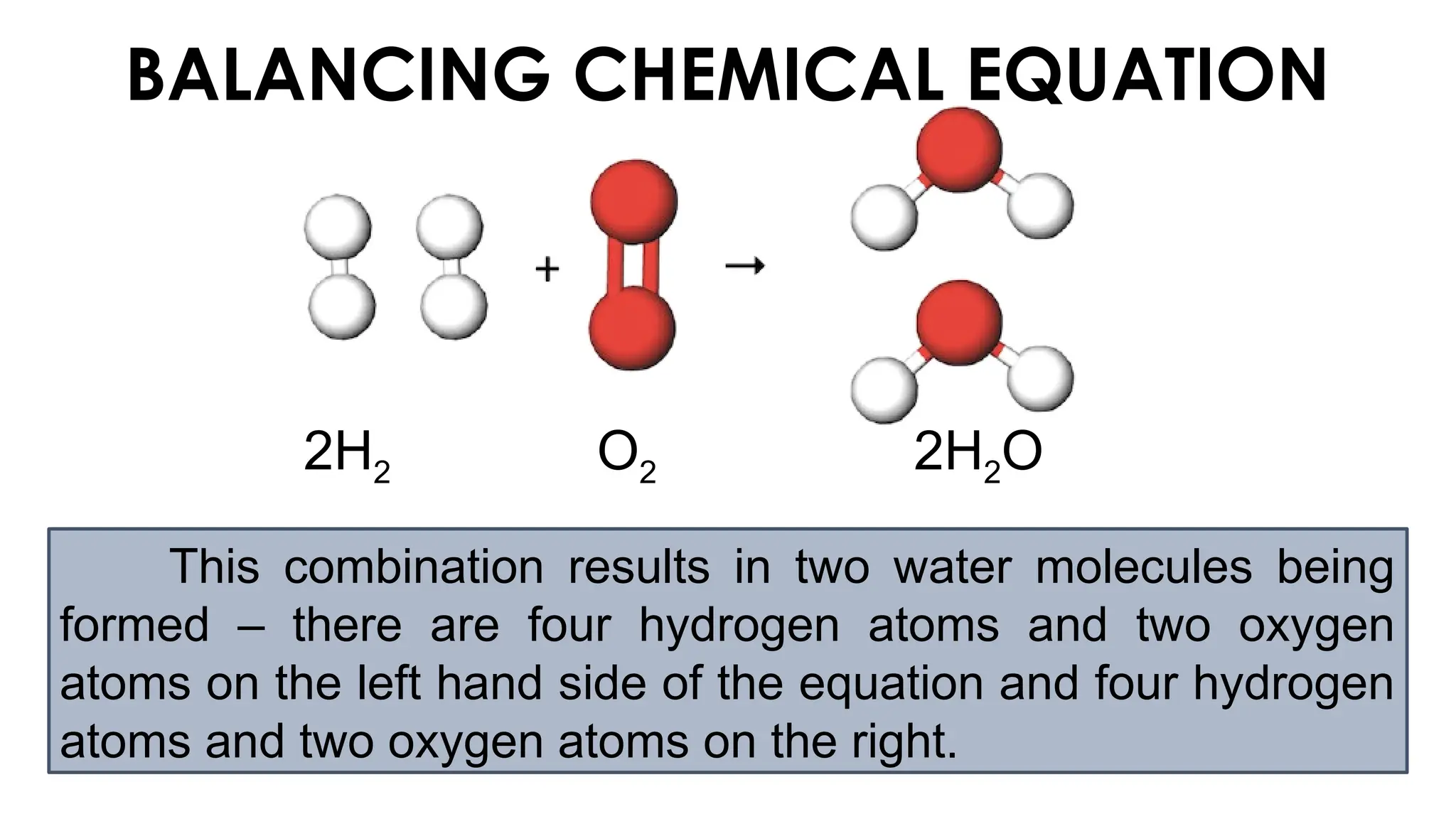
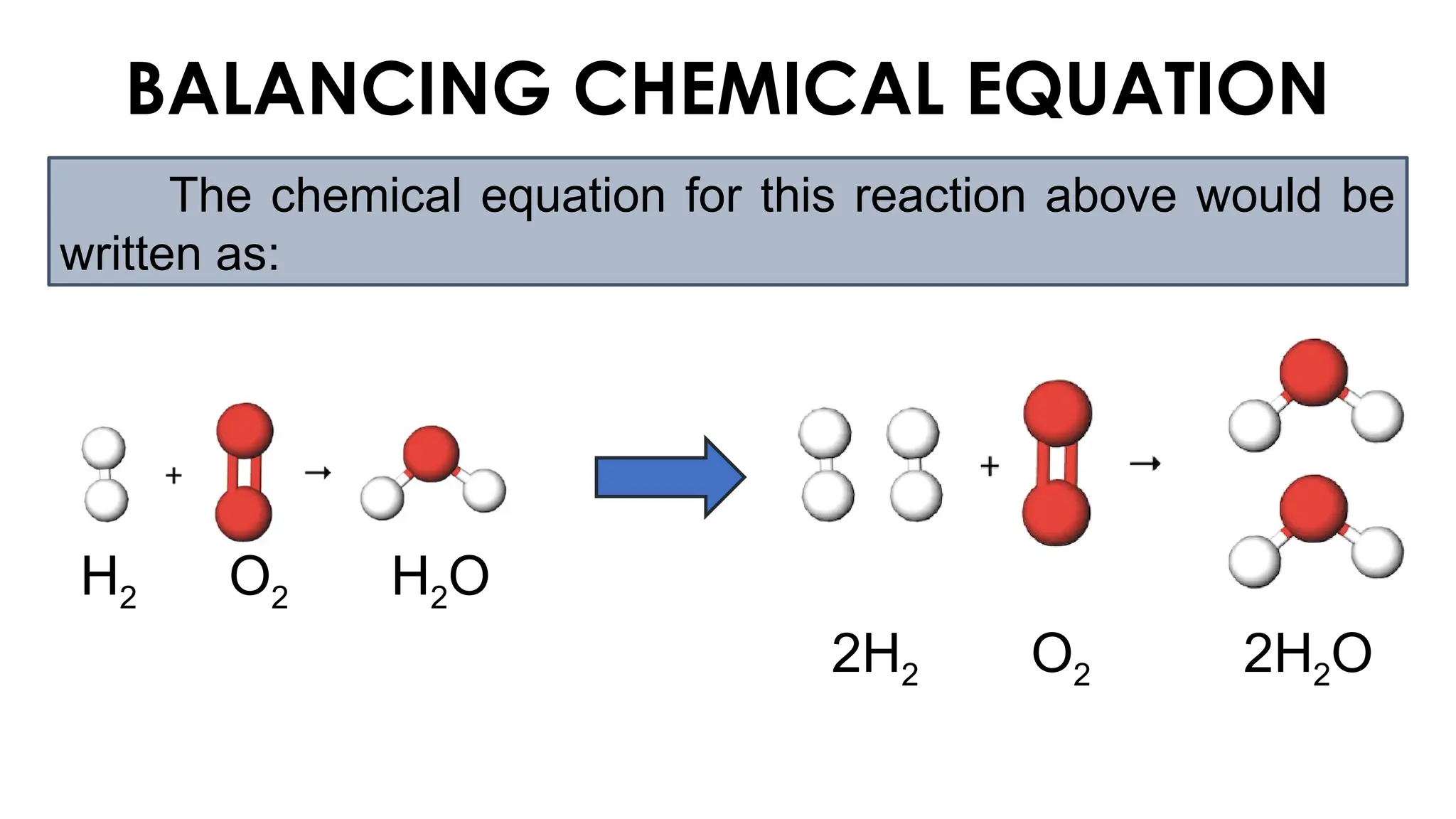
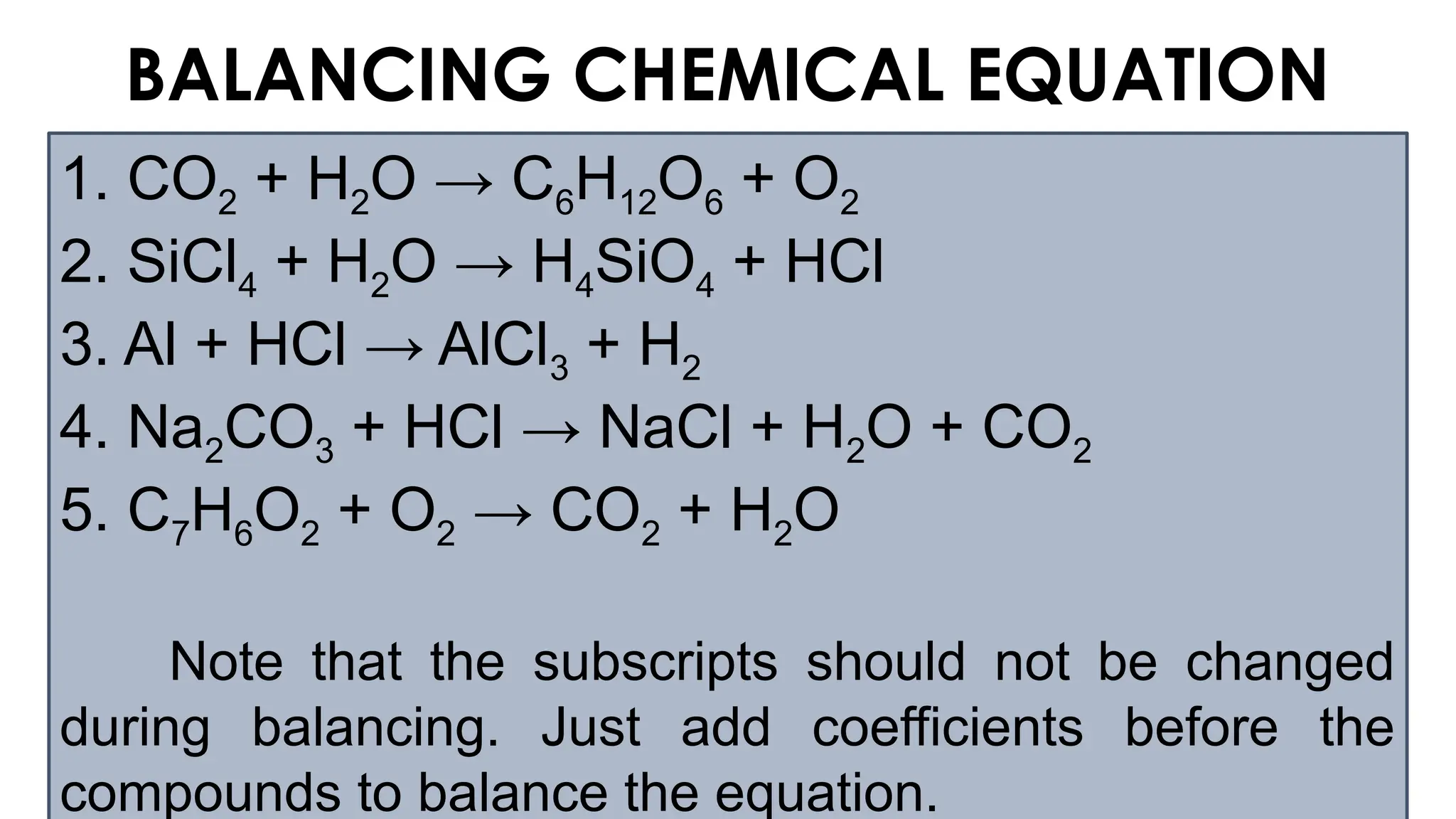
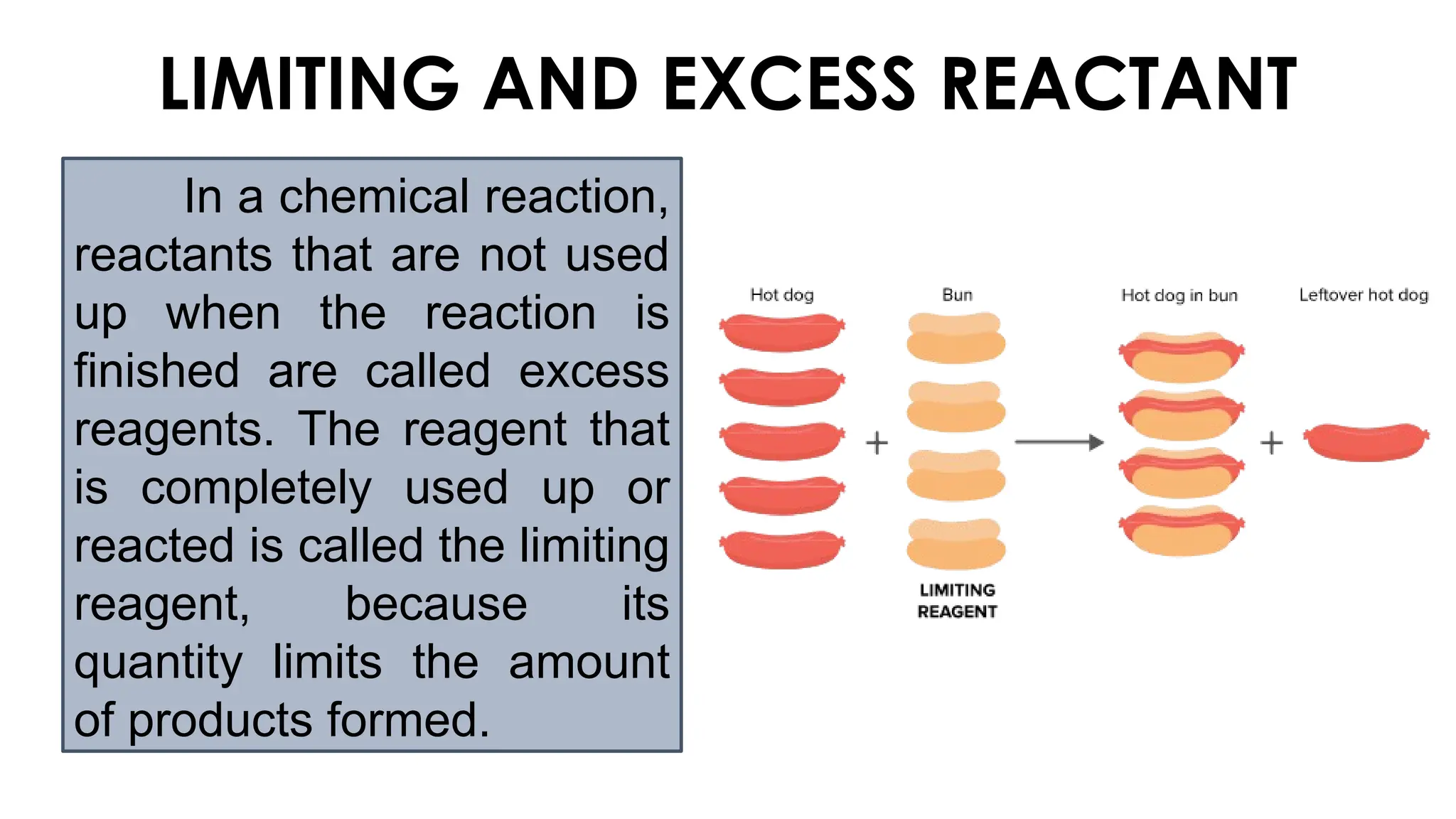
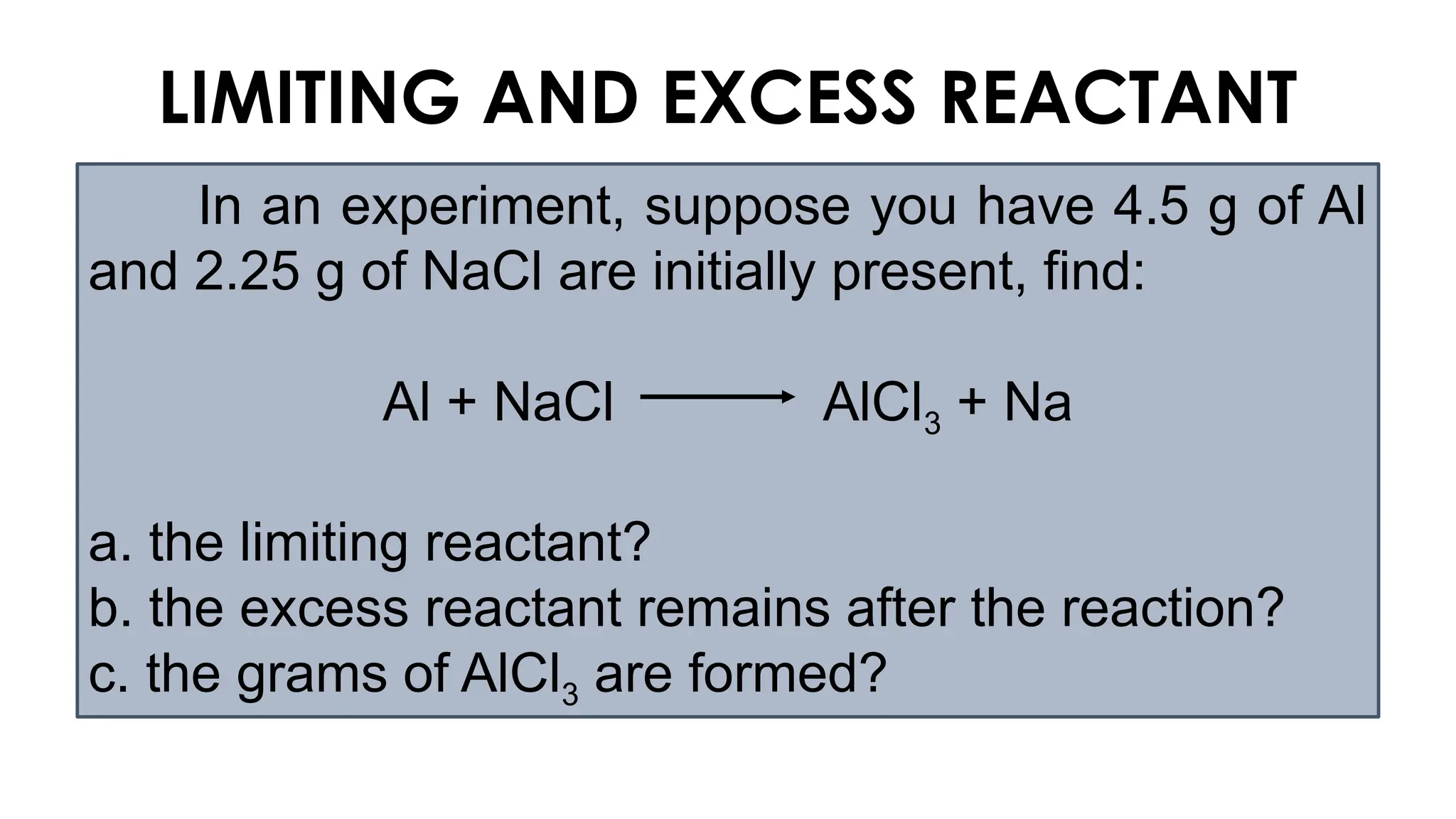
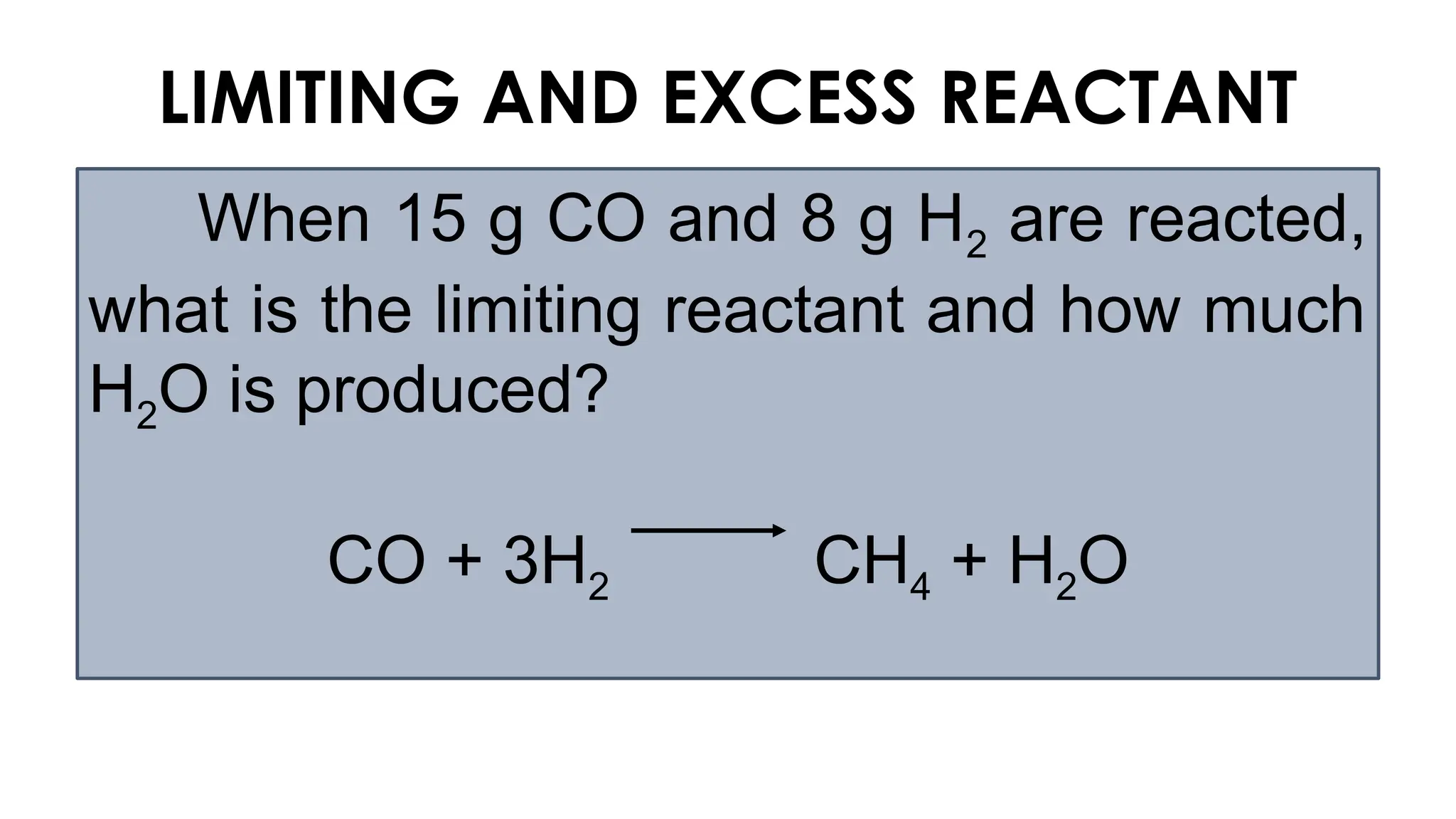The document explains the concept of balancing chemical equations, emphasizing the conservation of mass and that atoms are neither created nor destroyed. It introduces limiting and excess reactants, where the limiting reactant determines the amount of product formed, while excess reactants remain after the reaction. Examples of balancing equations and determining limiting reagents are provided for clarity.