Embed presentation
Downloaded 58 times
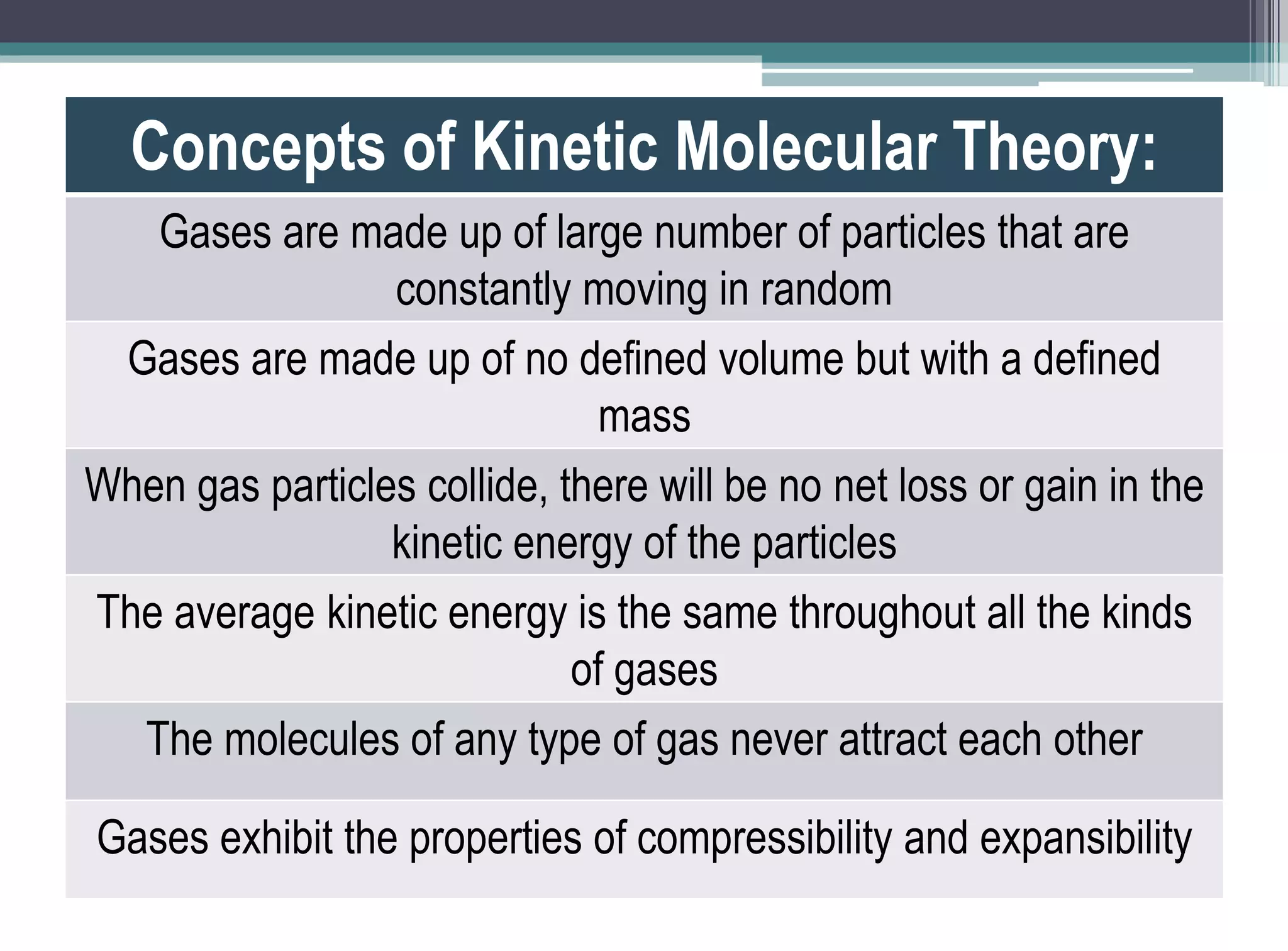
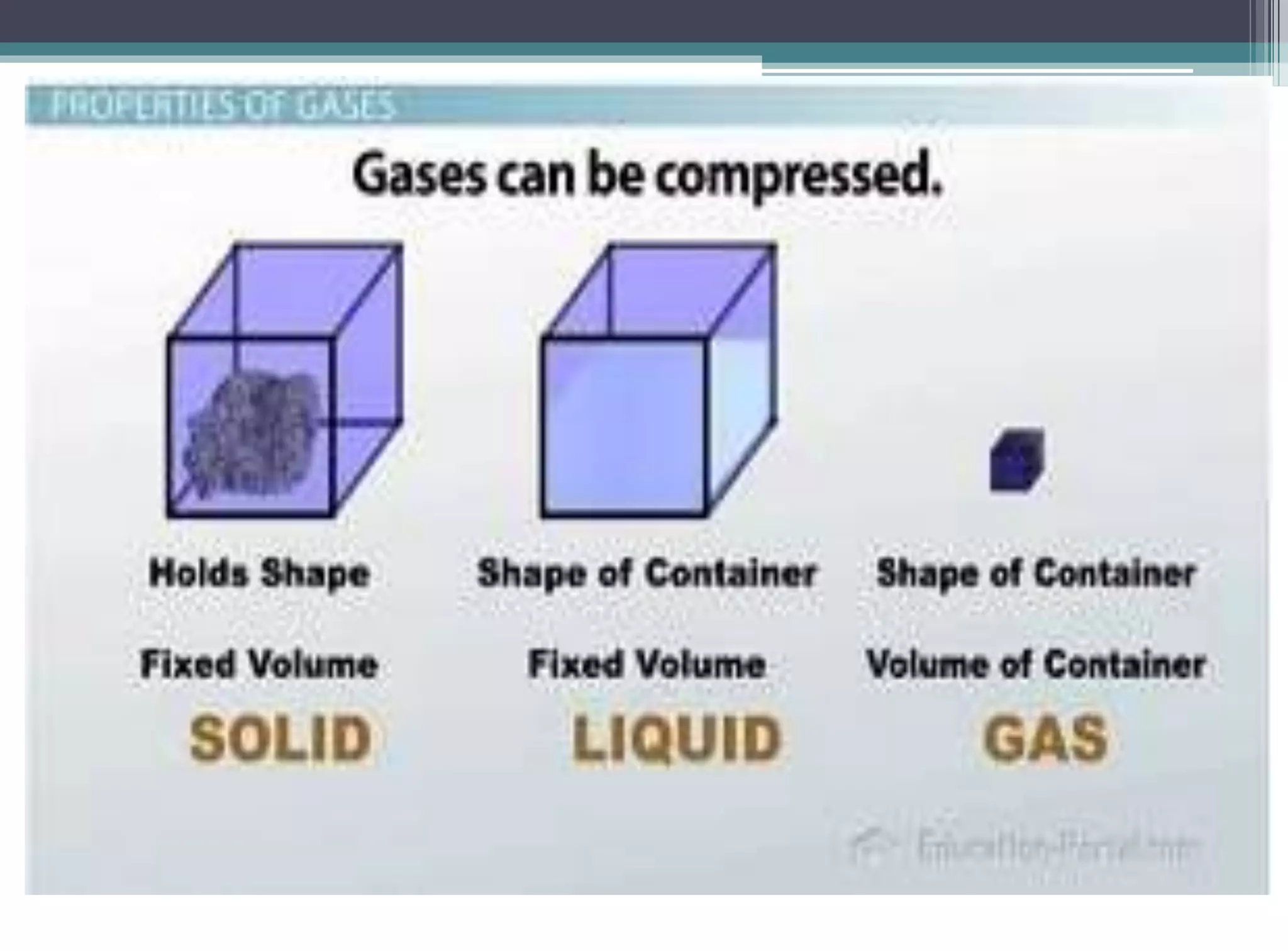
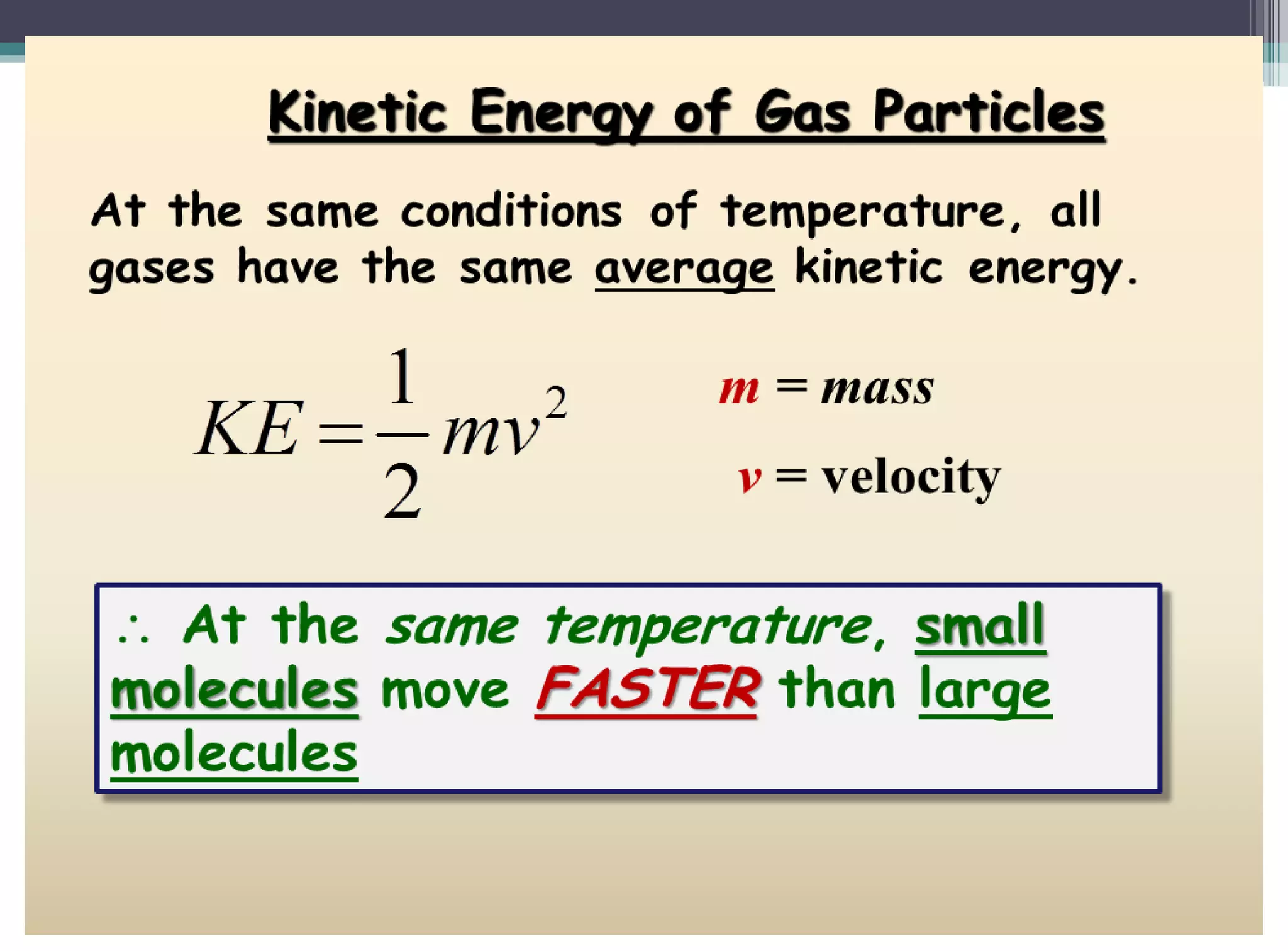
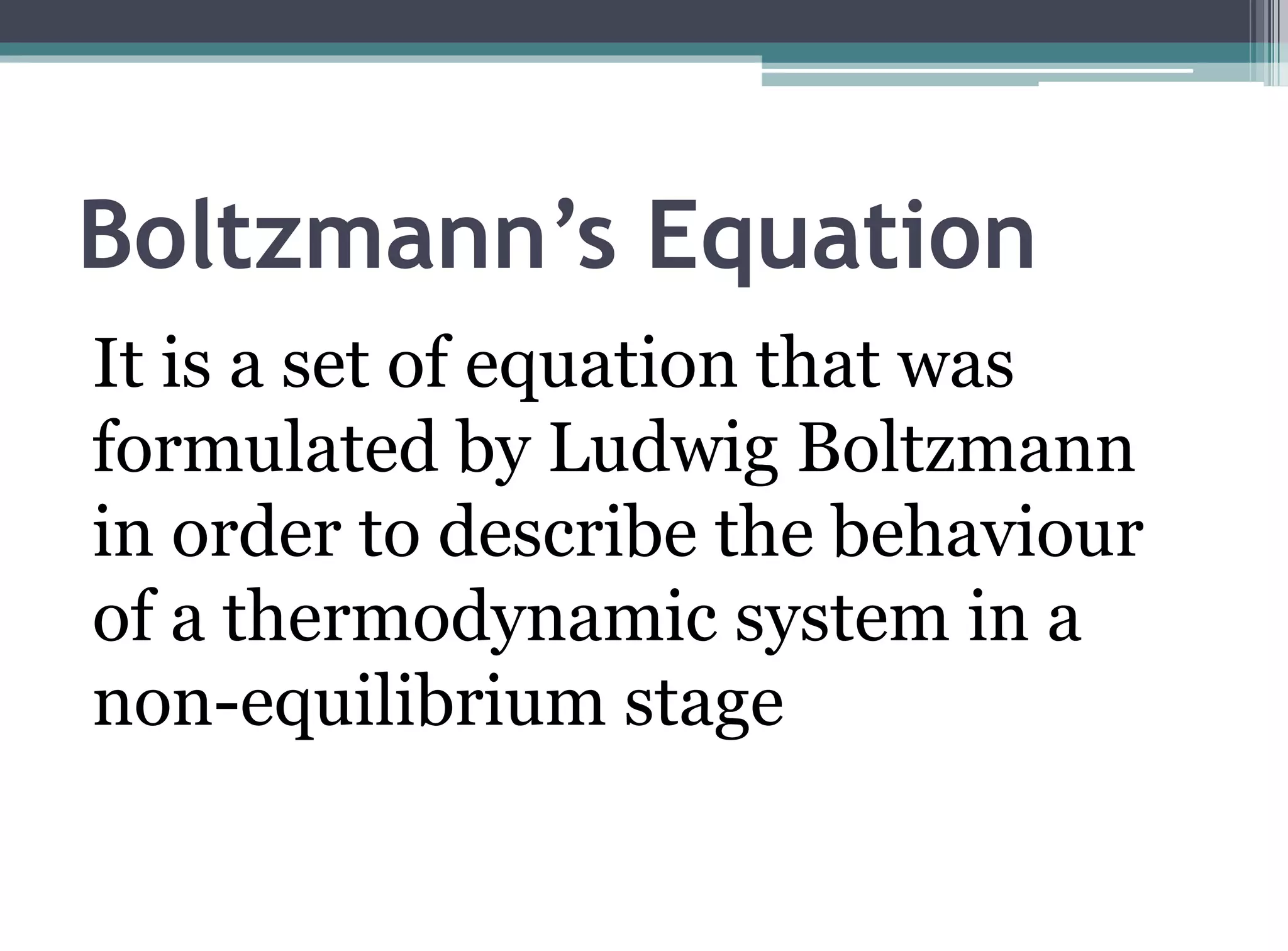

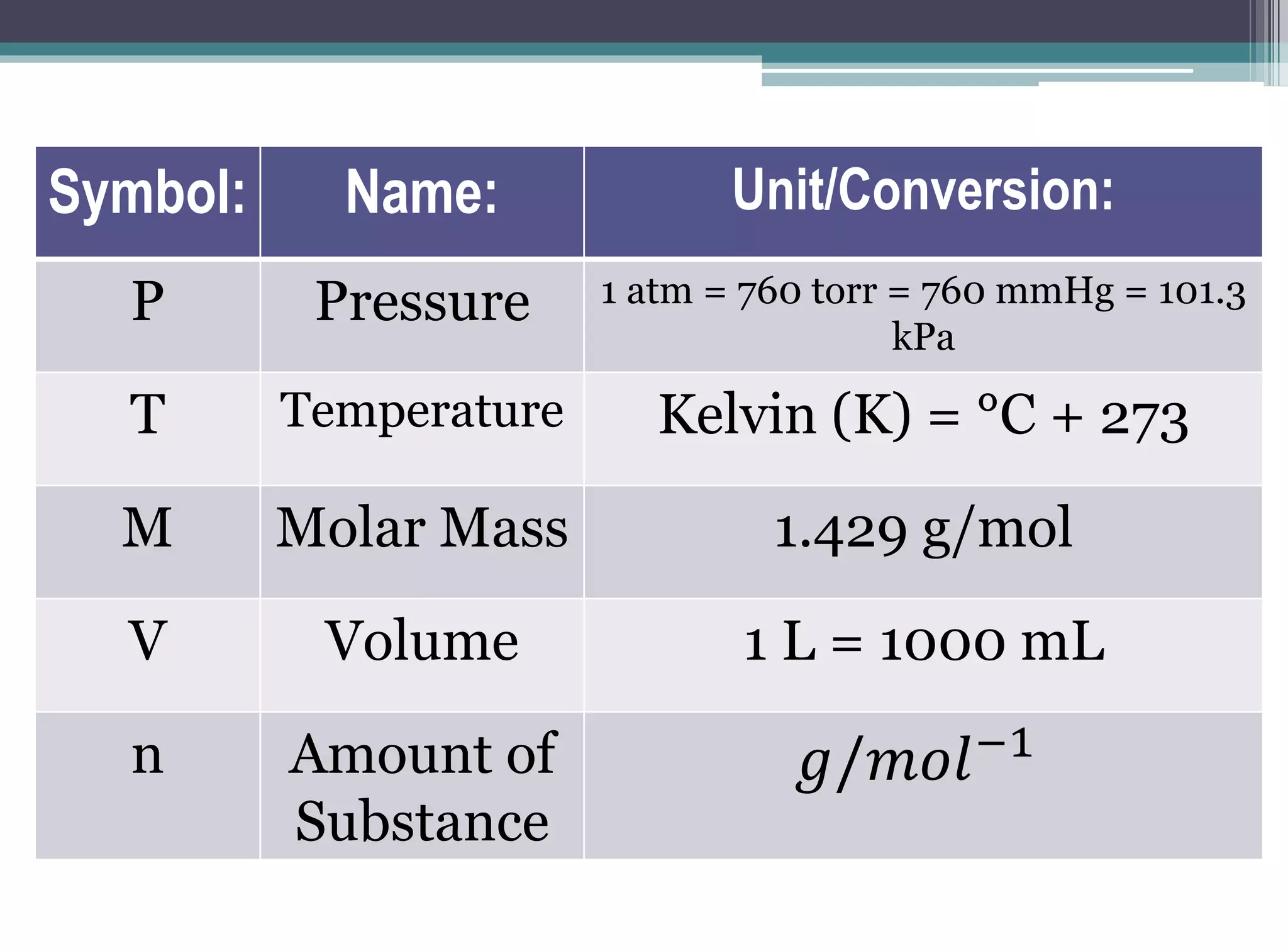
The kinetic molecular theory of gases explains gas behavior and macroscopic properties based on the motion of particles. Key concepts include random motion, no net energy loss in collisions, equal average kinetic energy, and the absence of molecular attraction. The theory also incorporates Boltzmann's equation and defines five major variables: pressure, temperature, molar mass, volume, and amount of substance.







