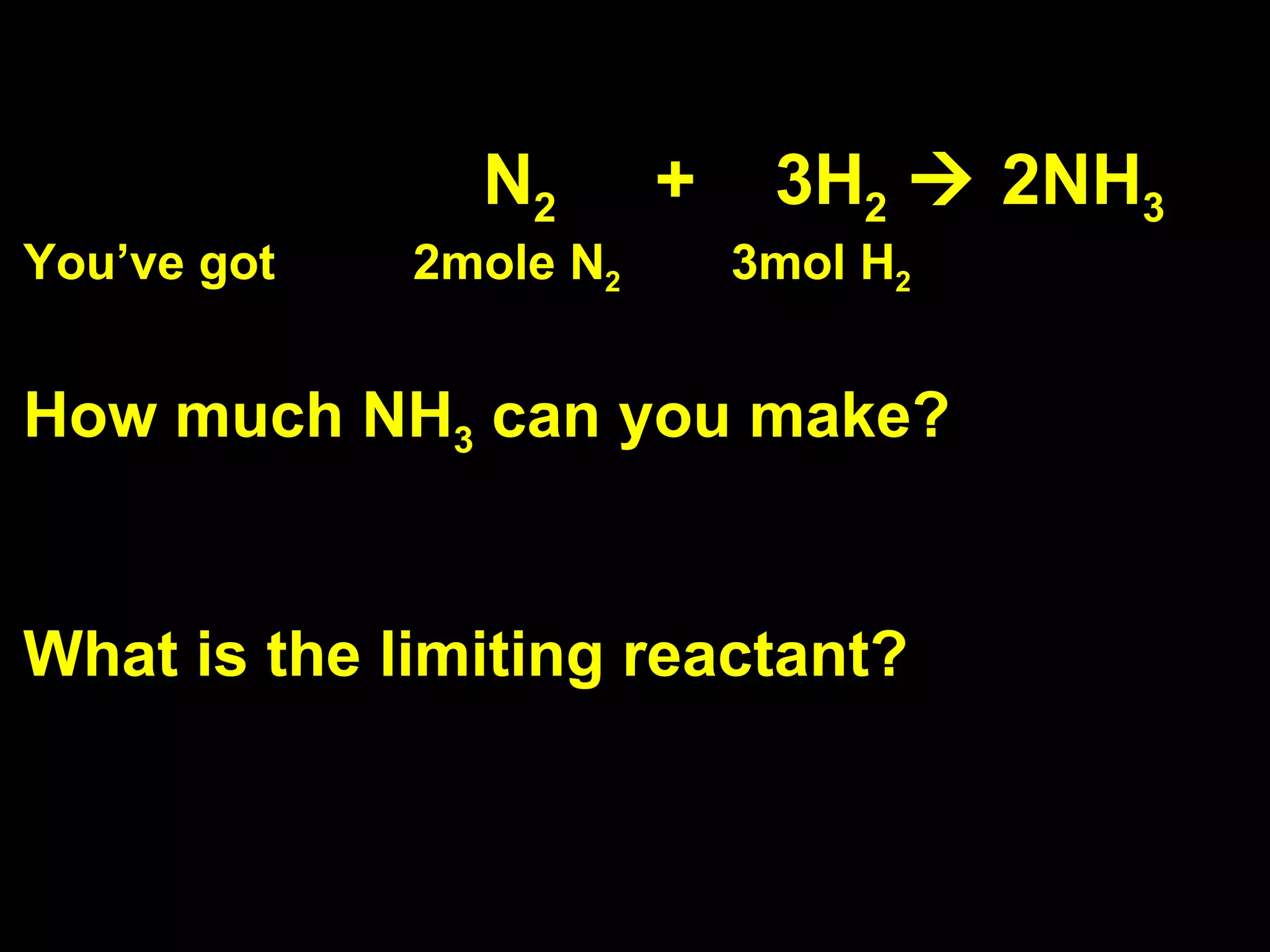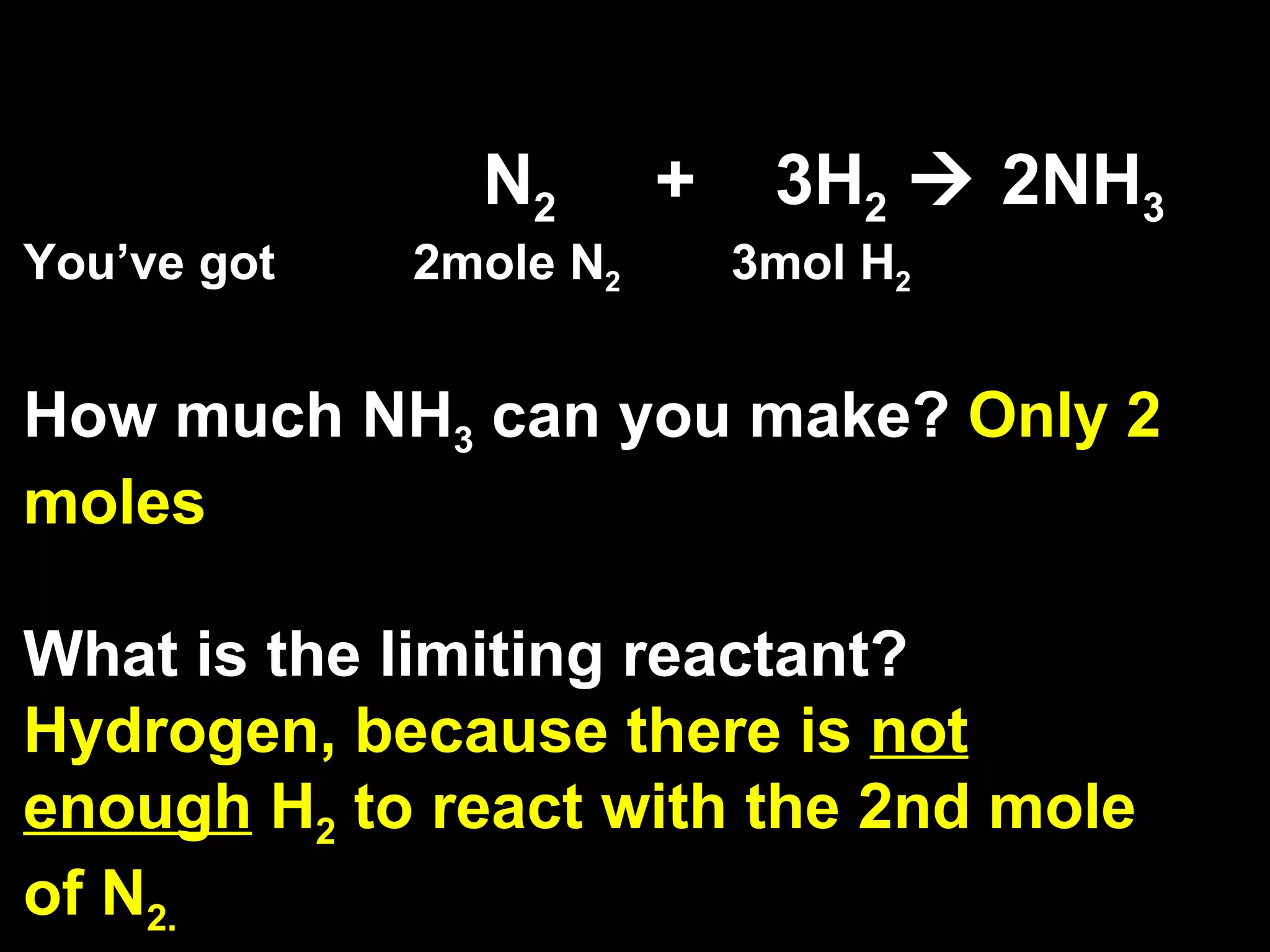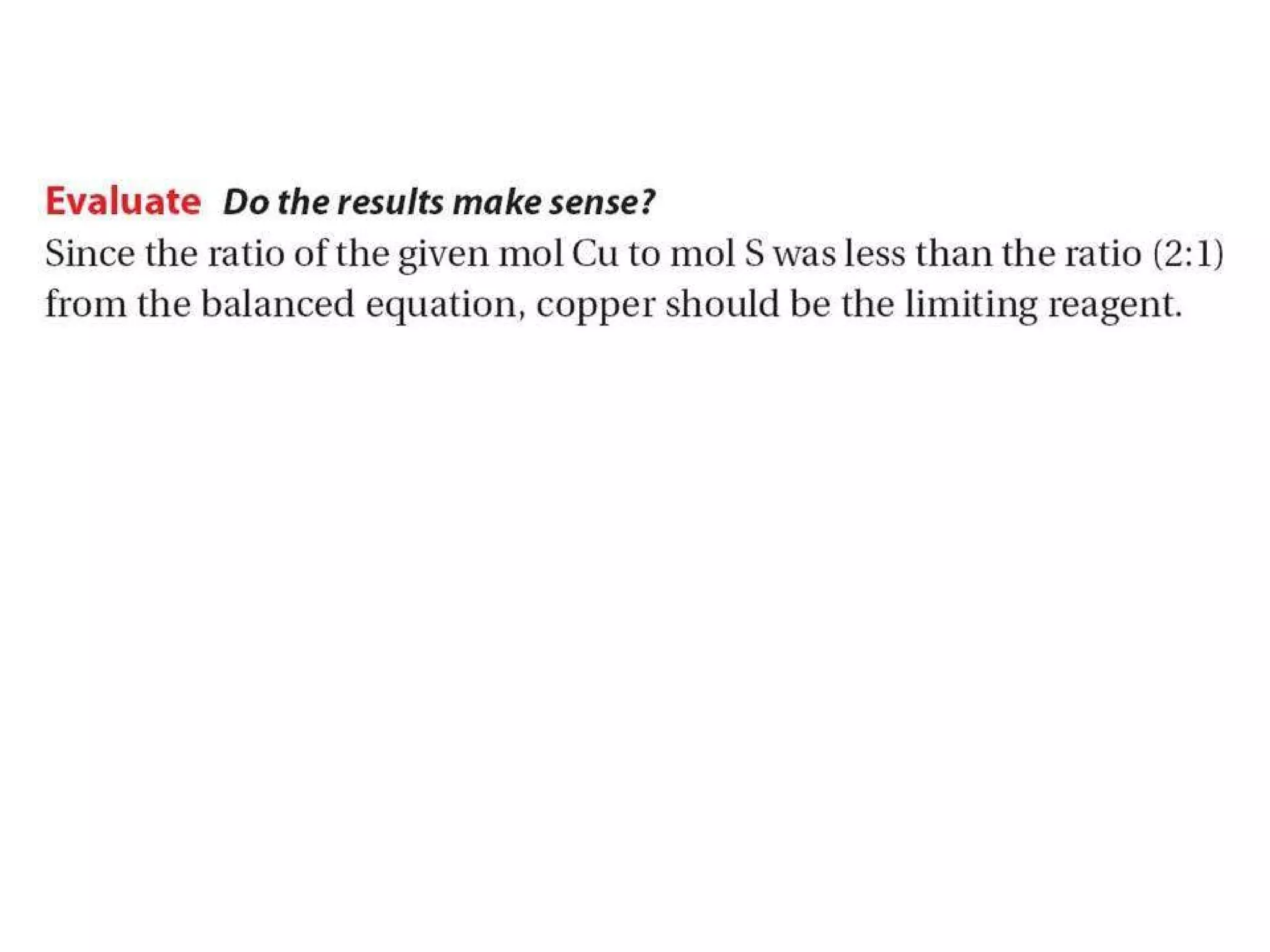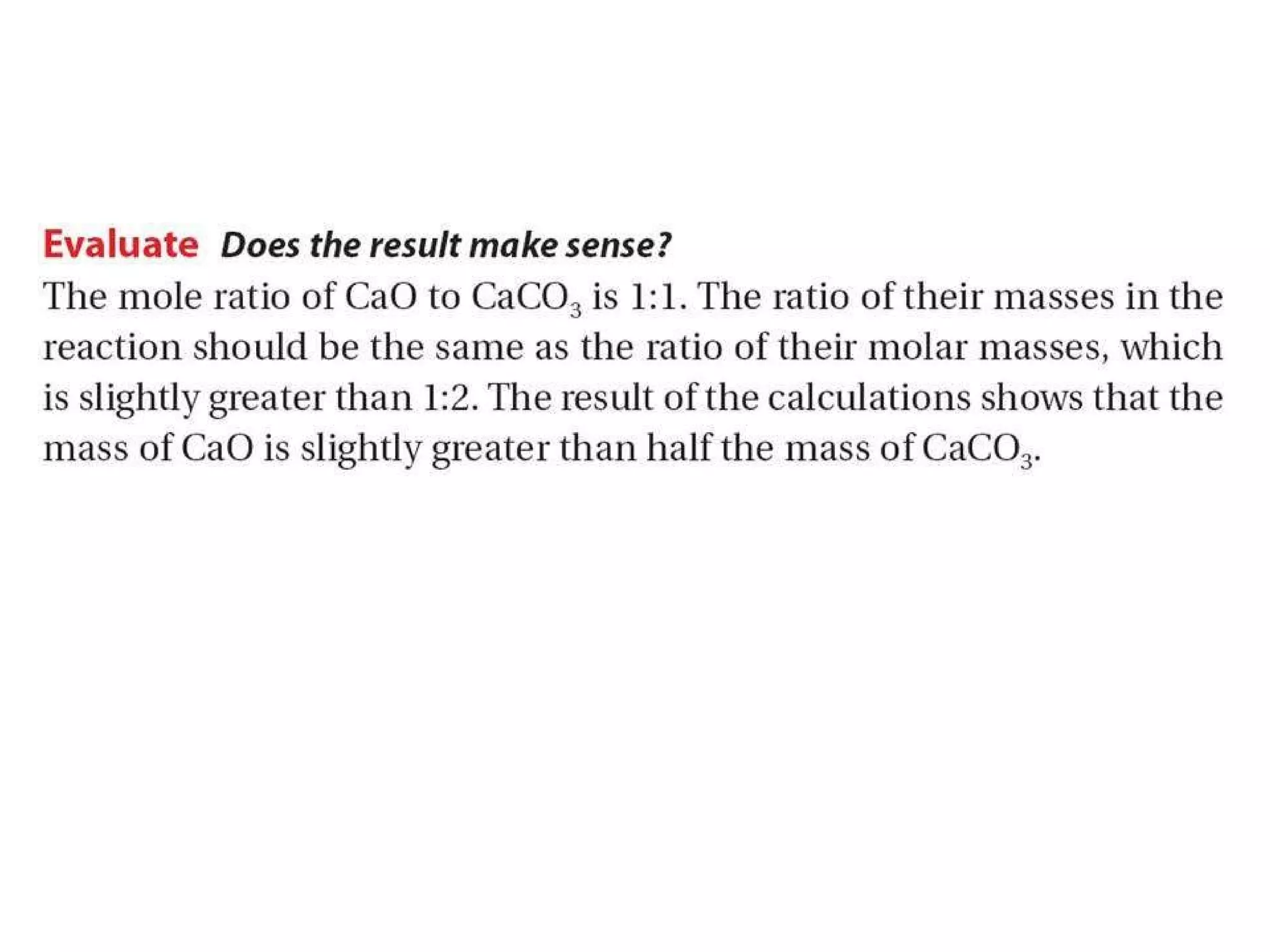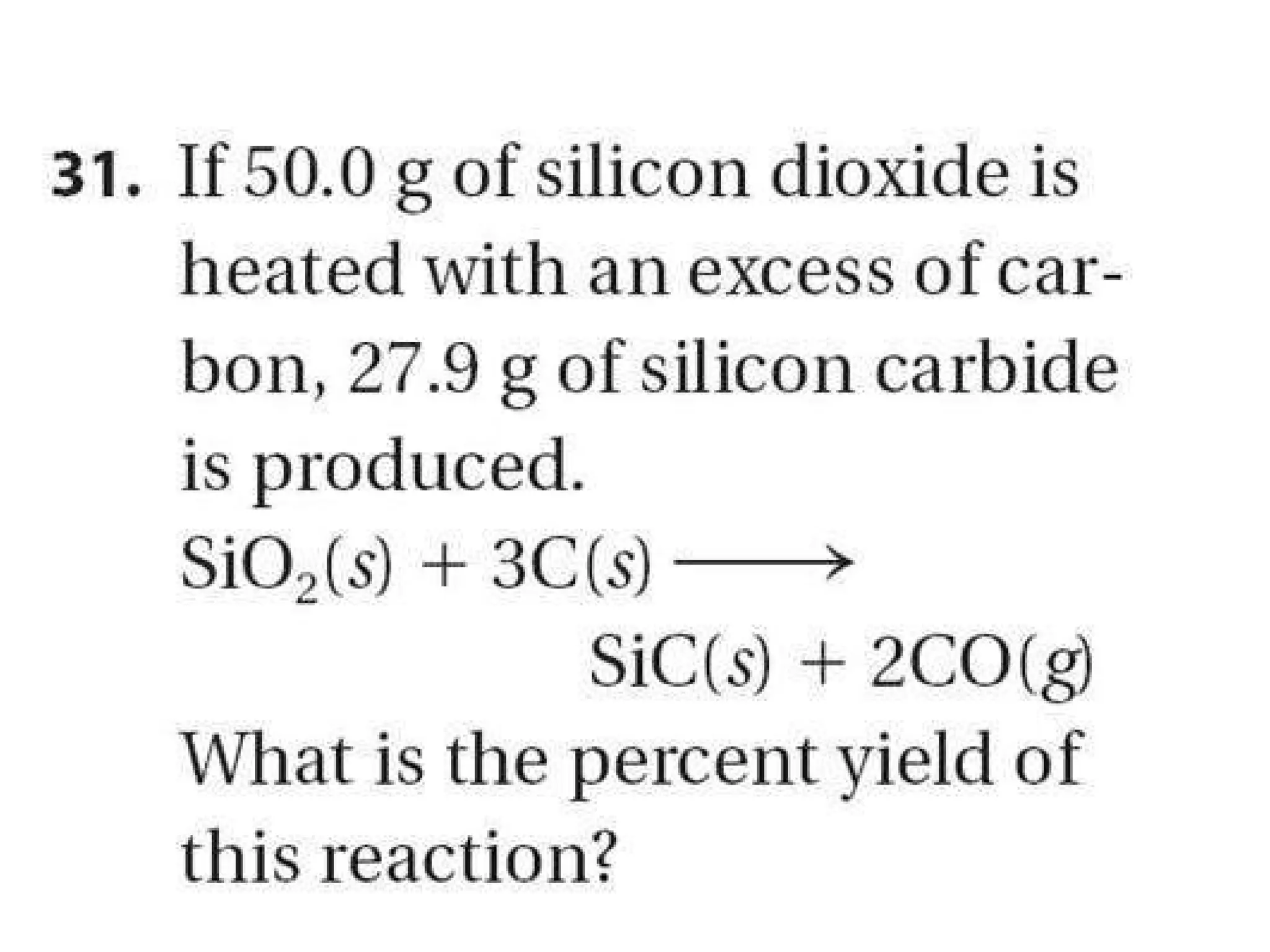The document discusses key concepts in chemistry including:
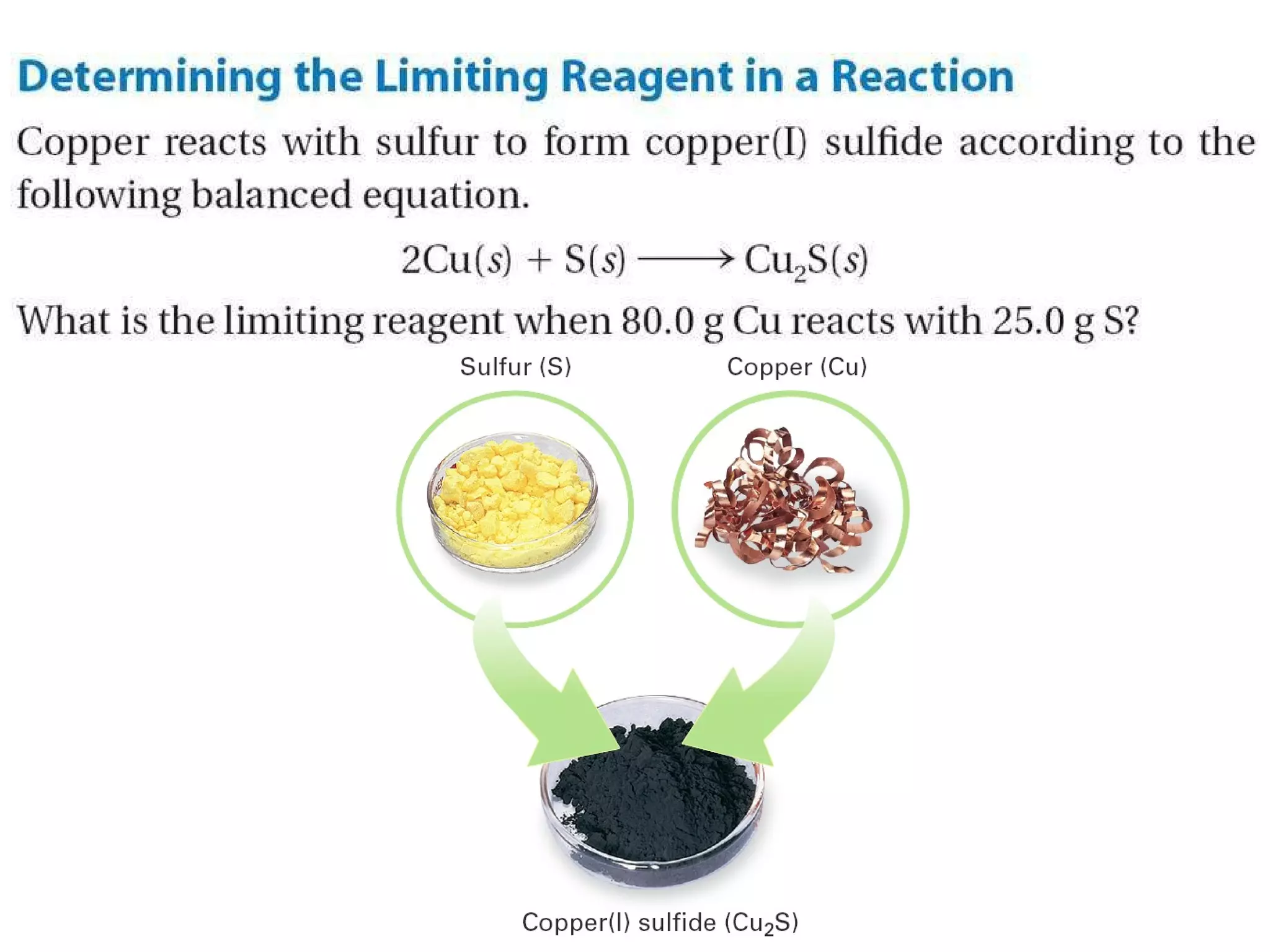
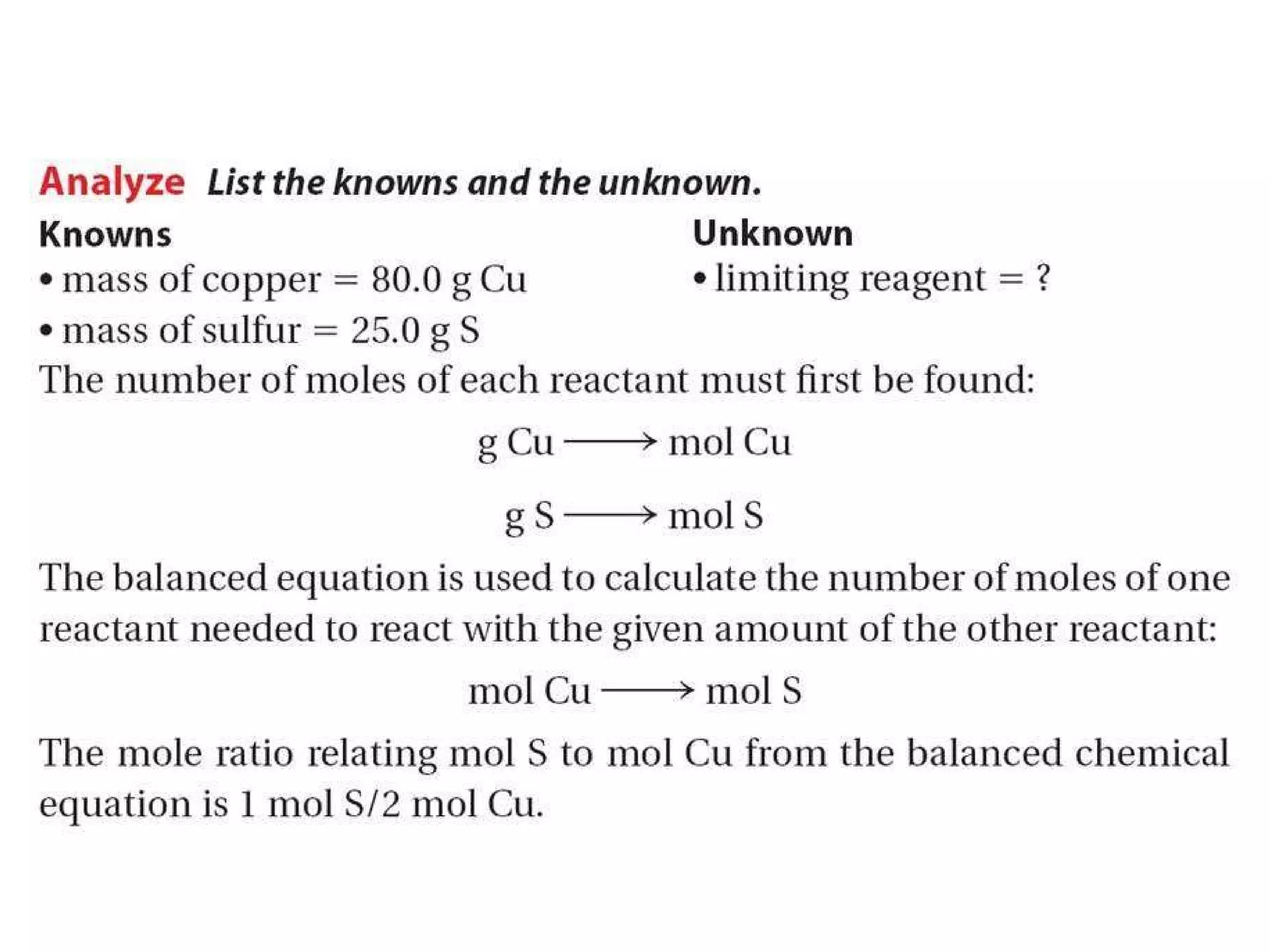
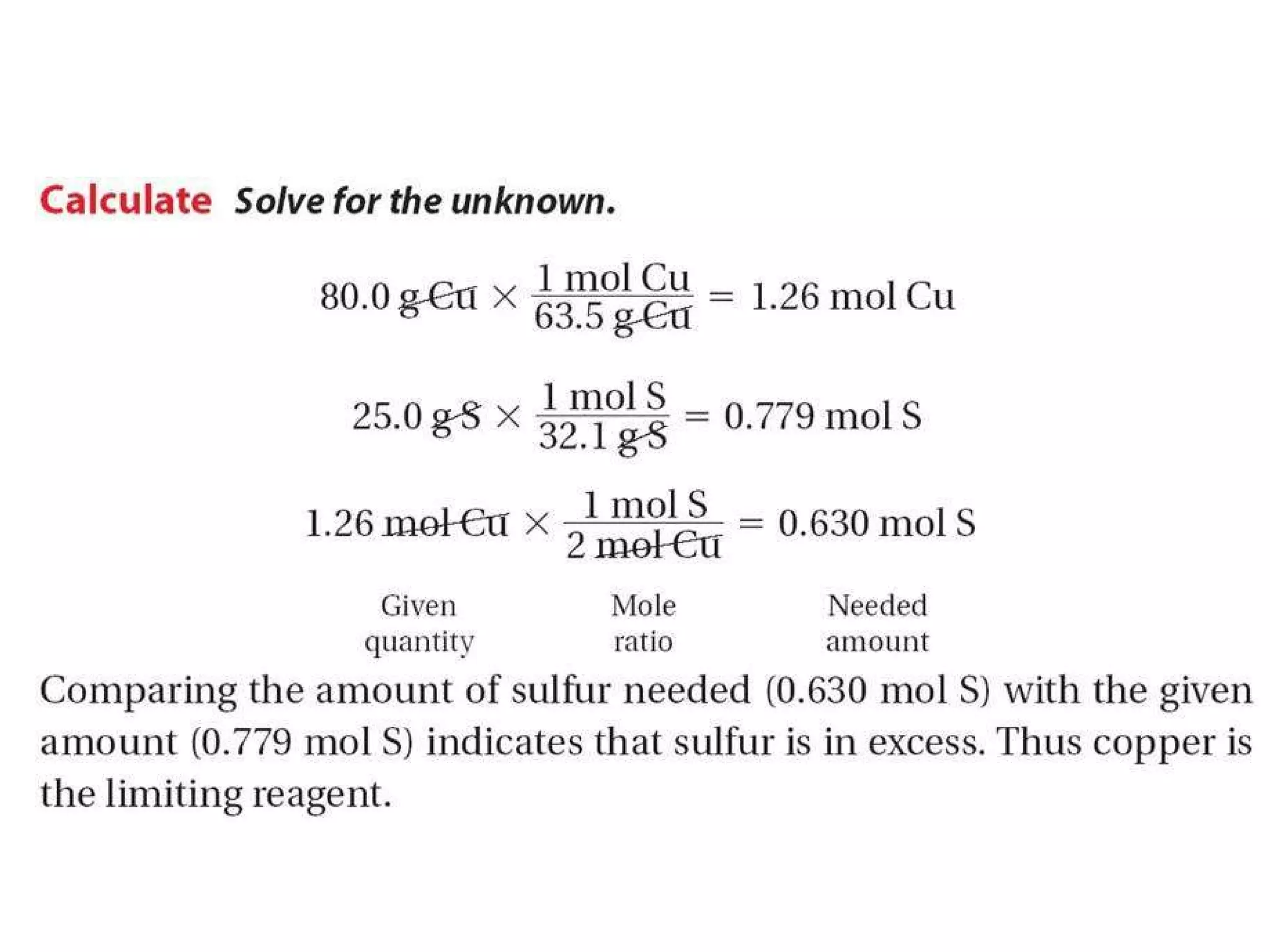
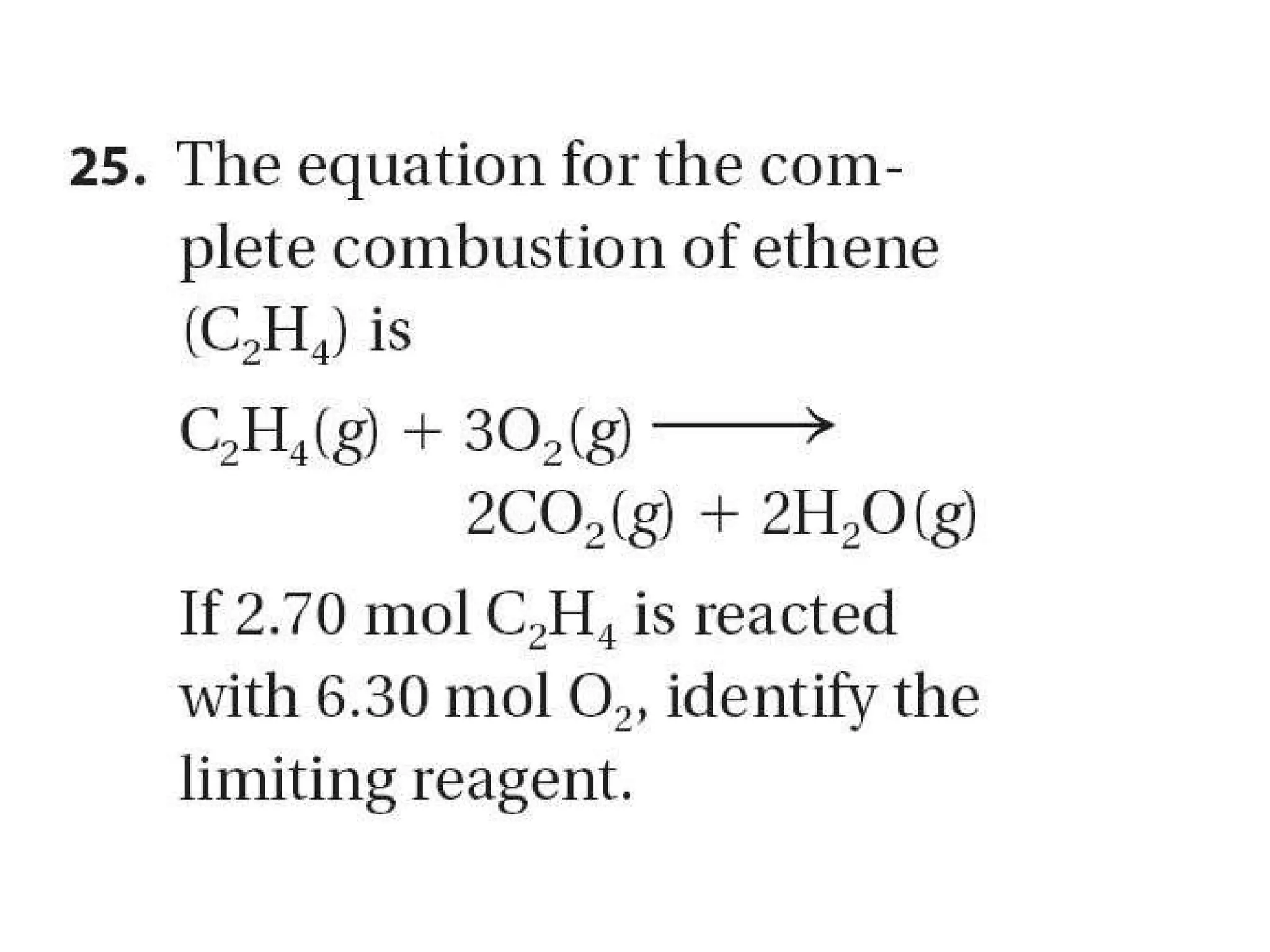
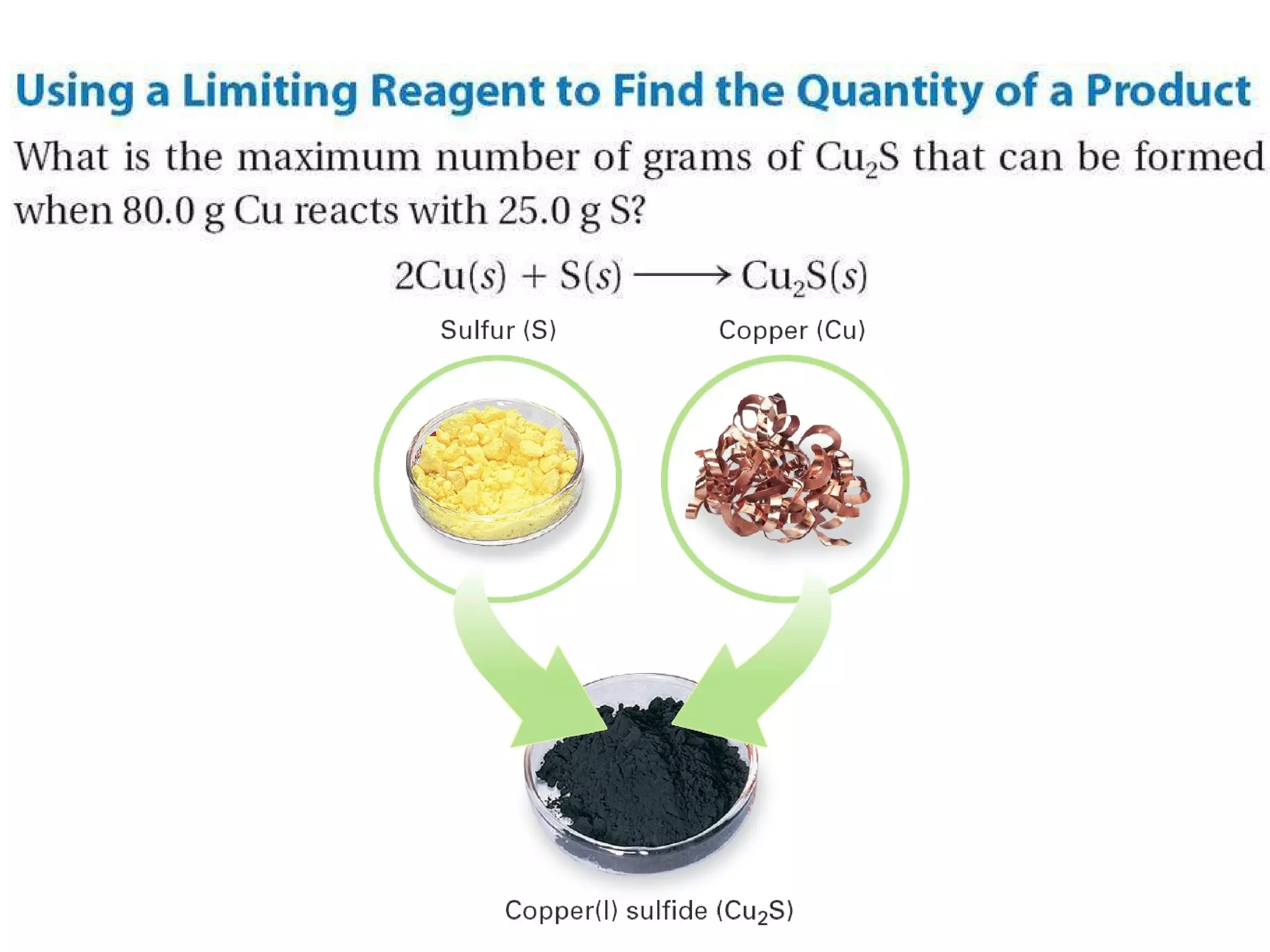
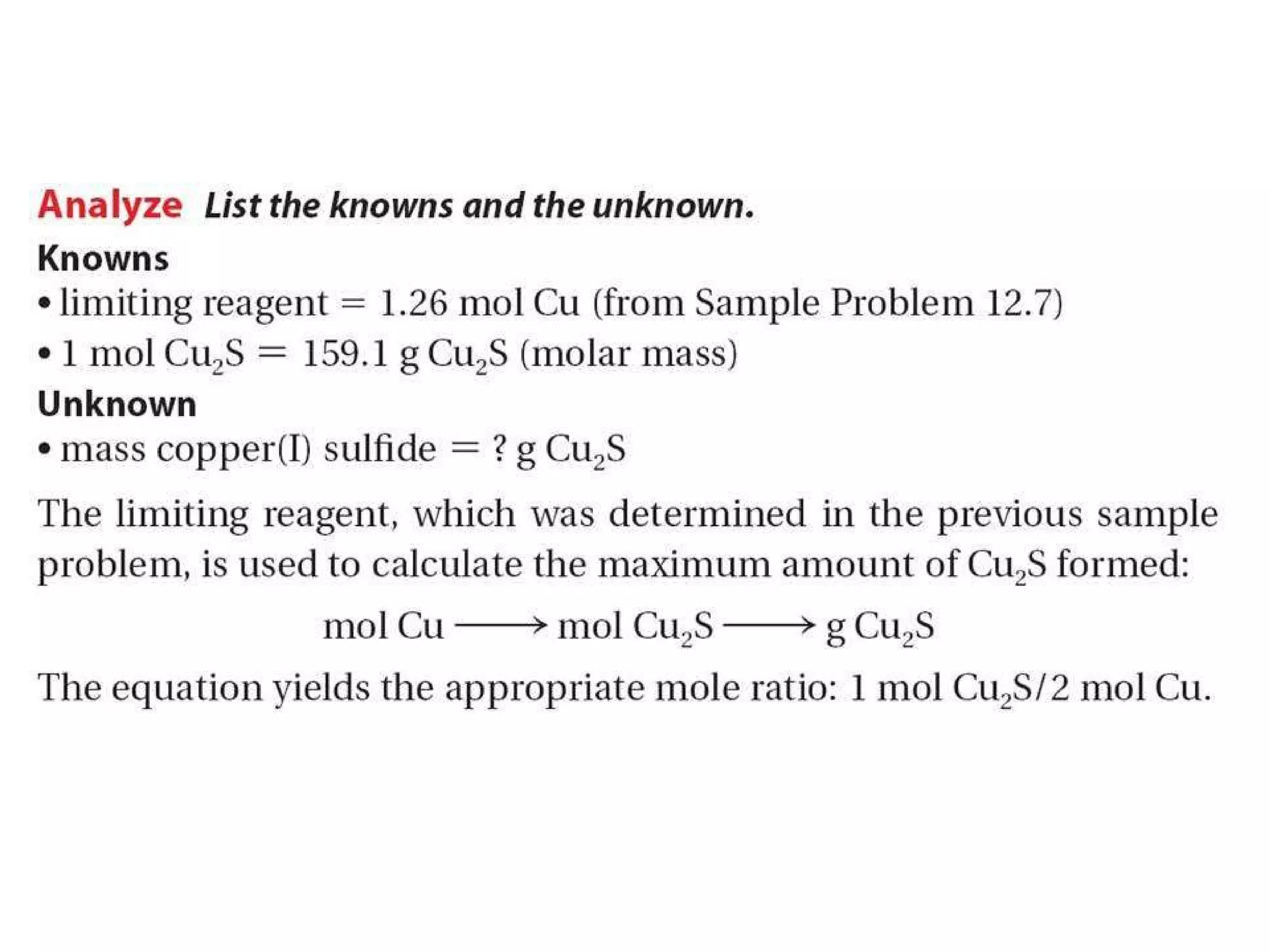
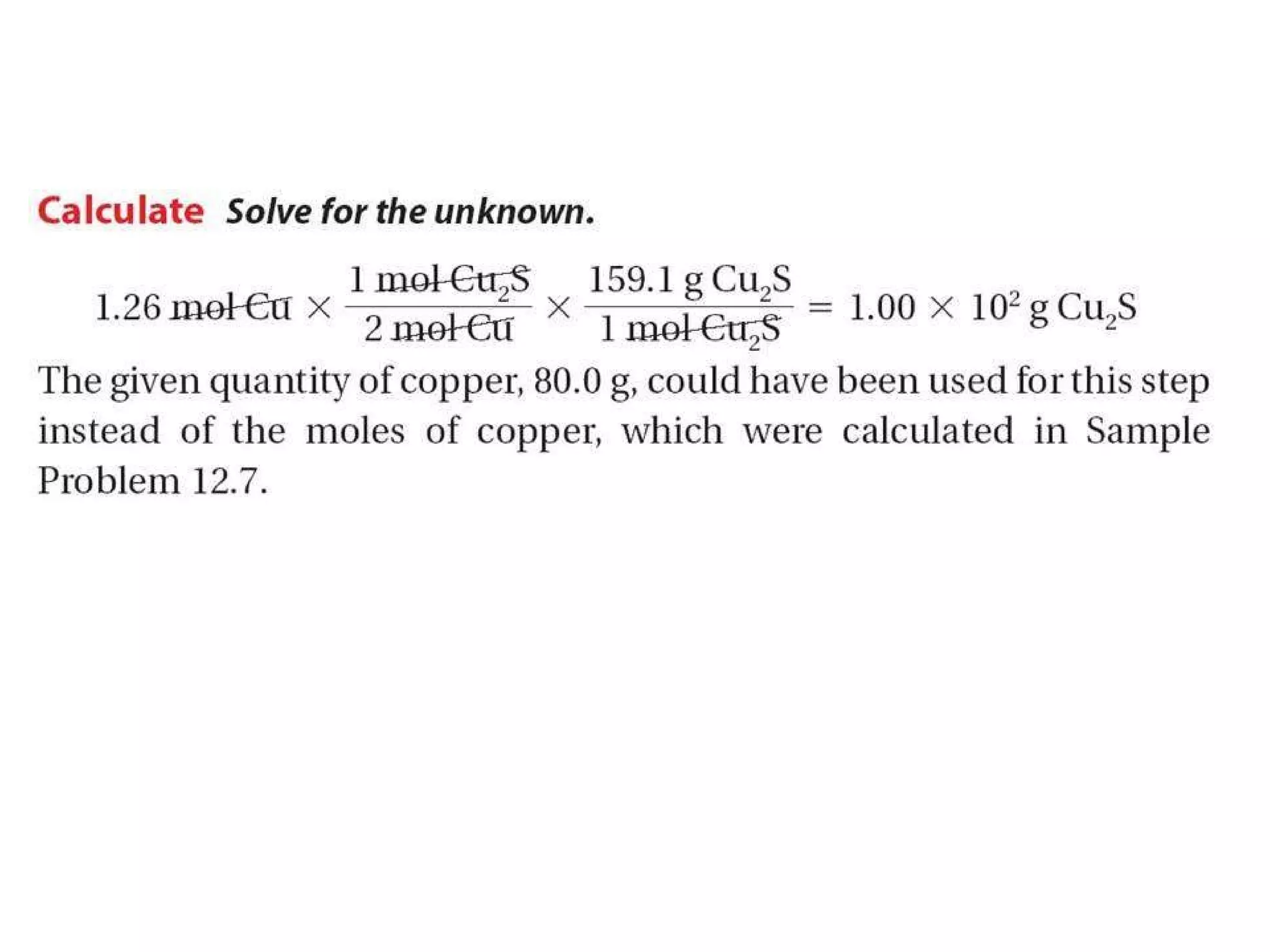
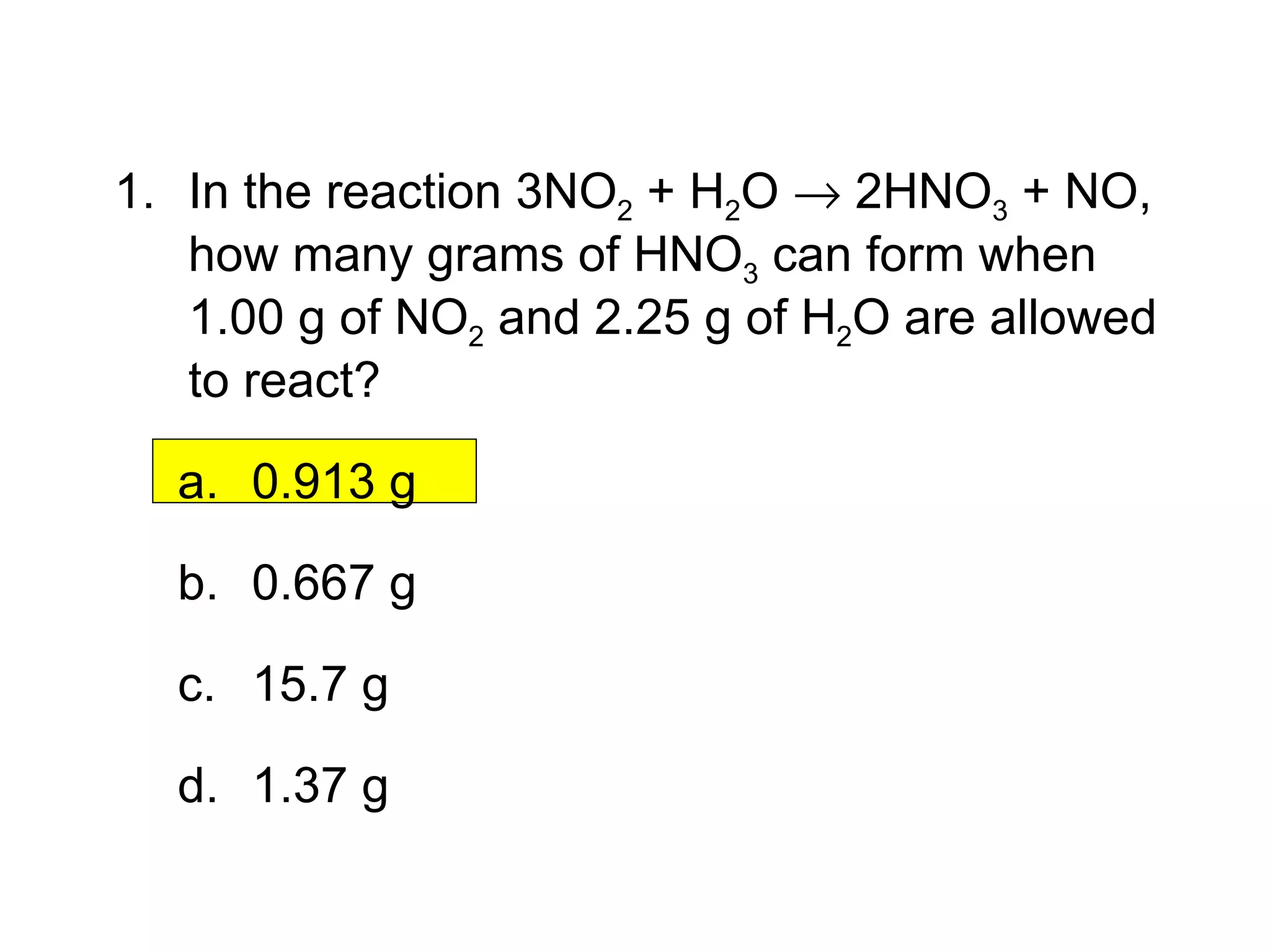
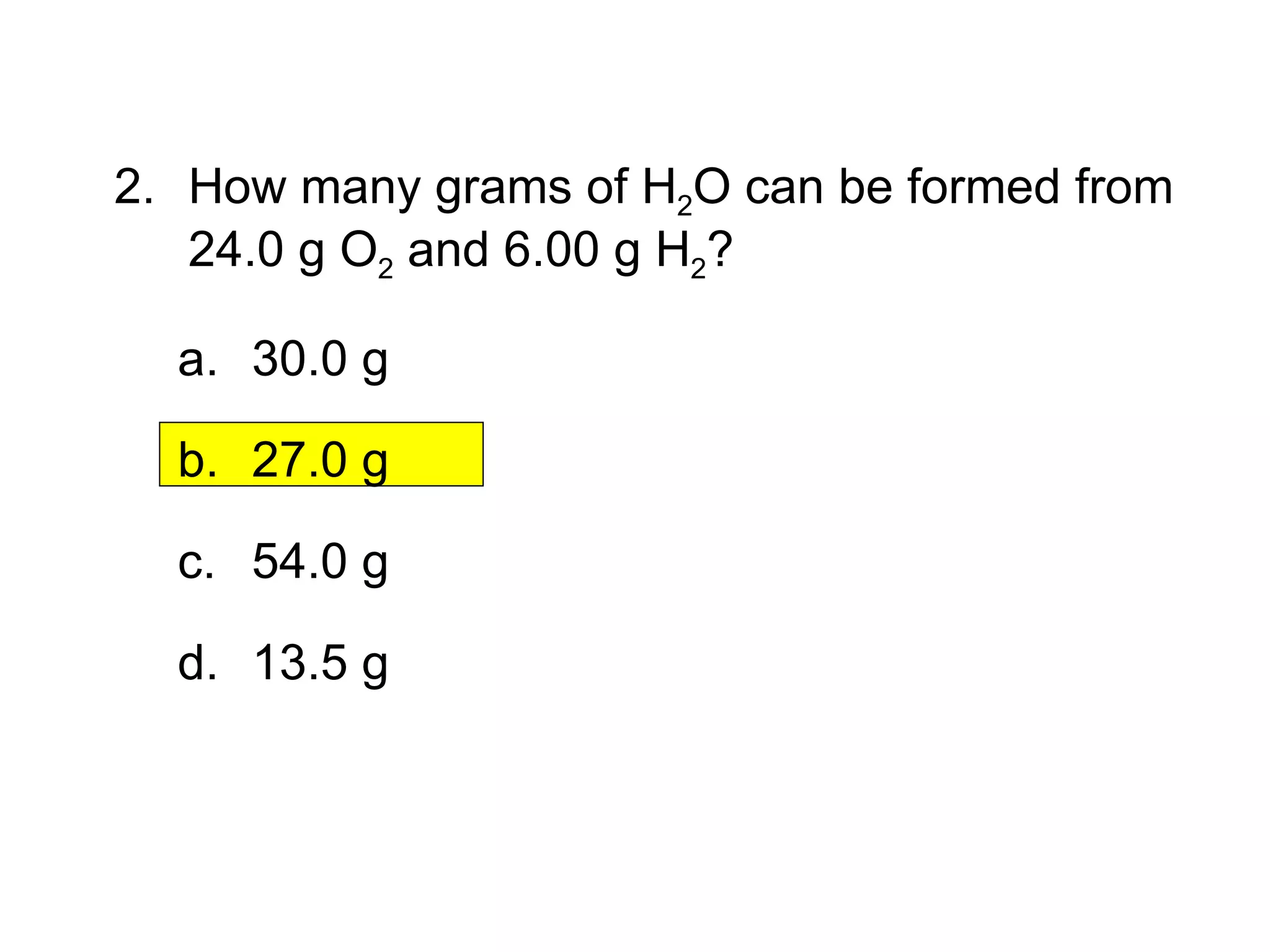
1) Limiting reagents and how the amount of one reactant determines how much product can be formed in a reaction.
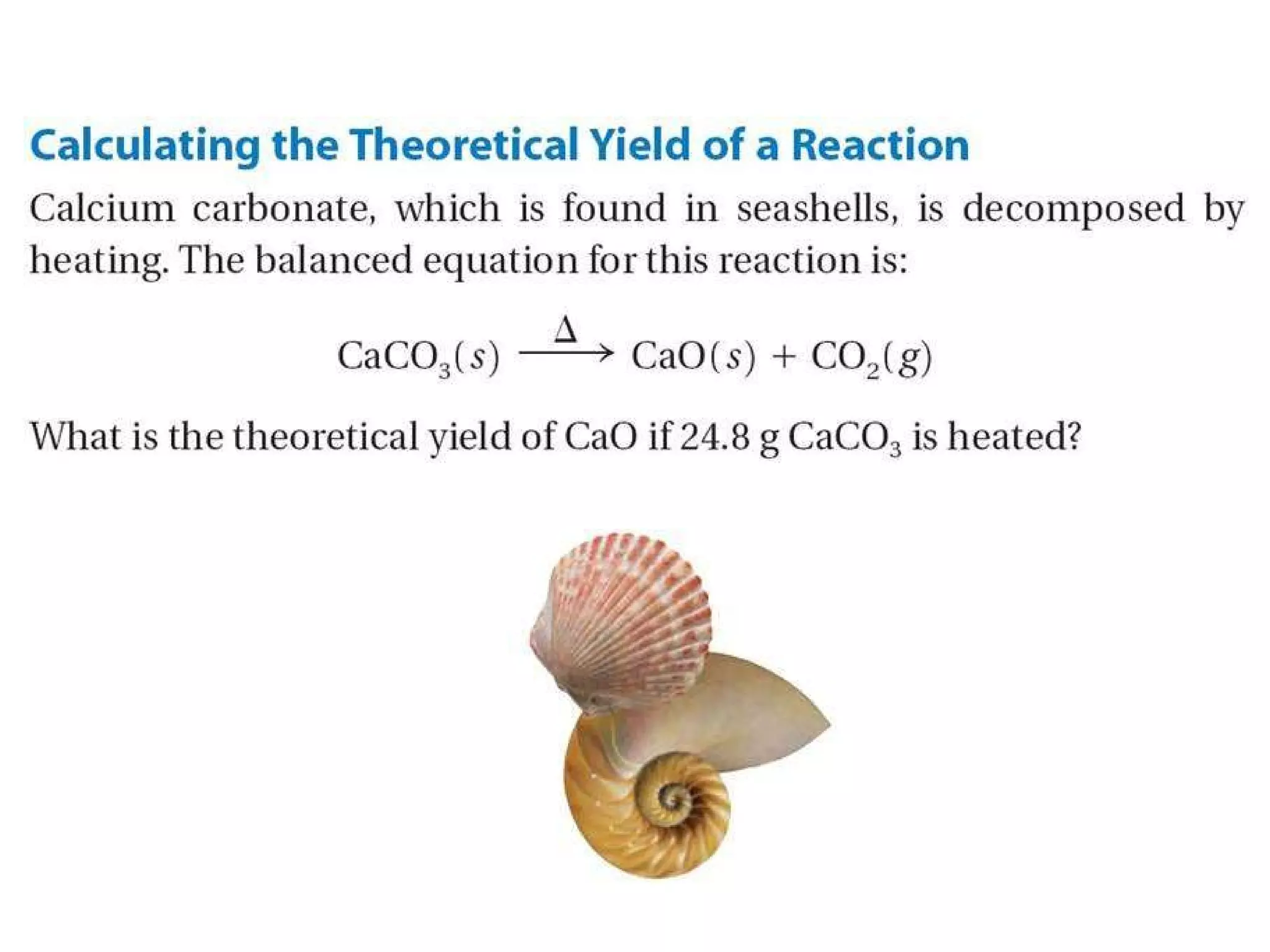
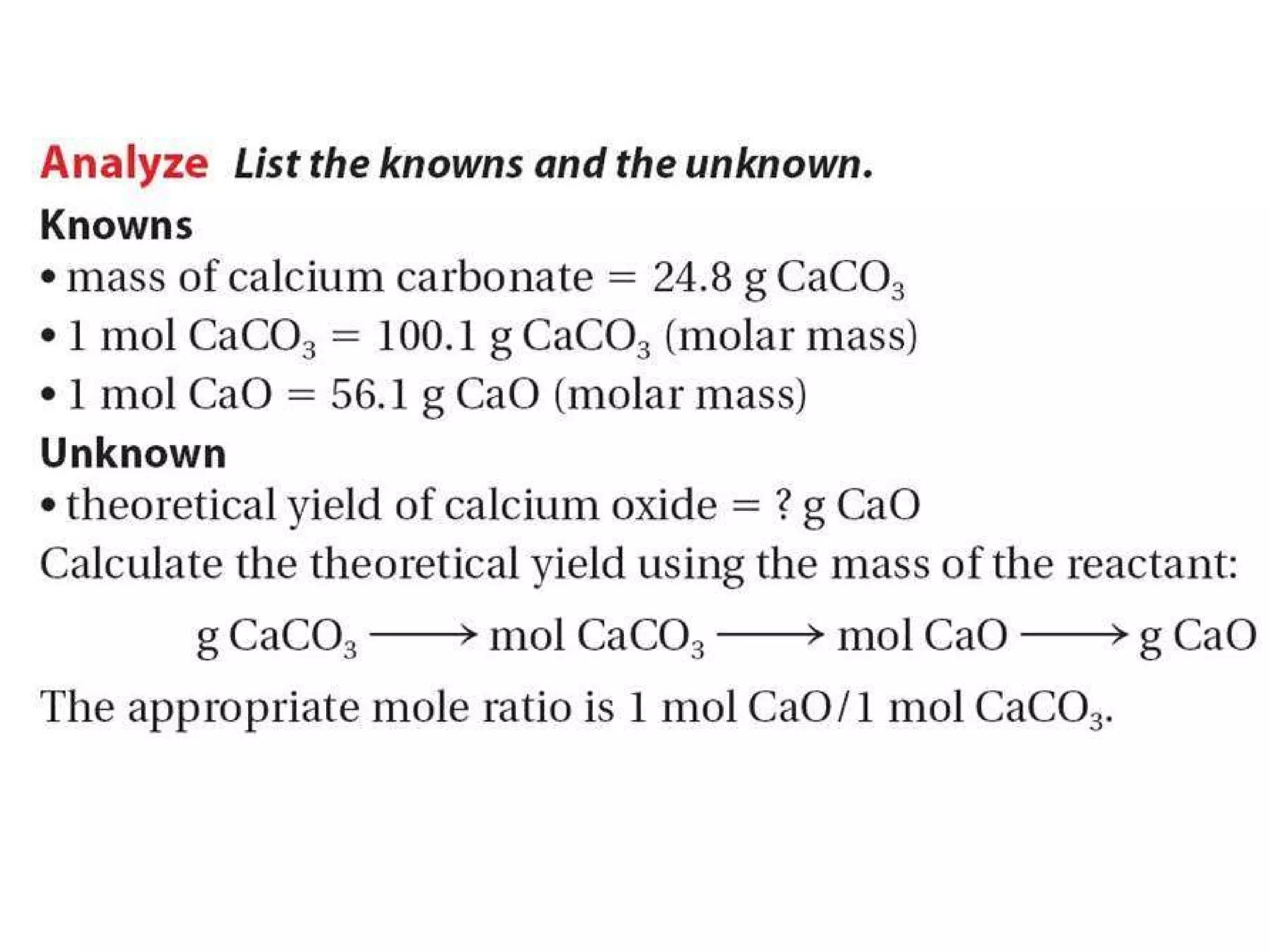
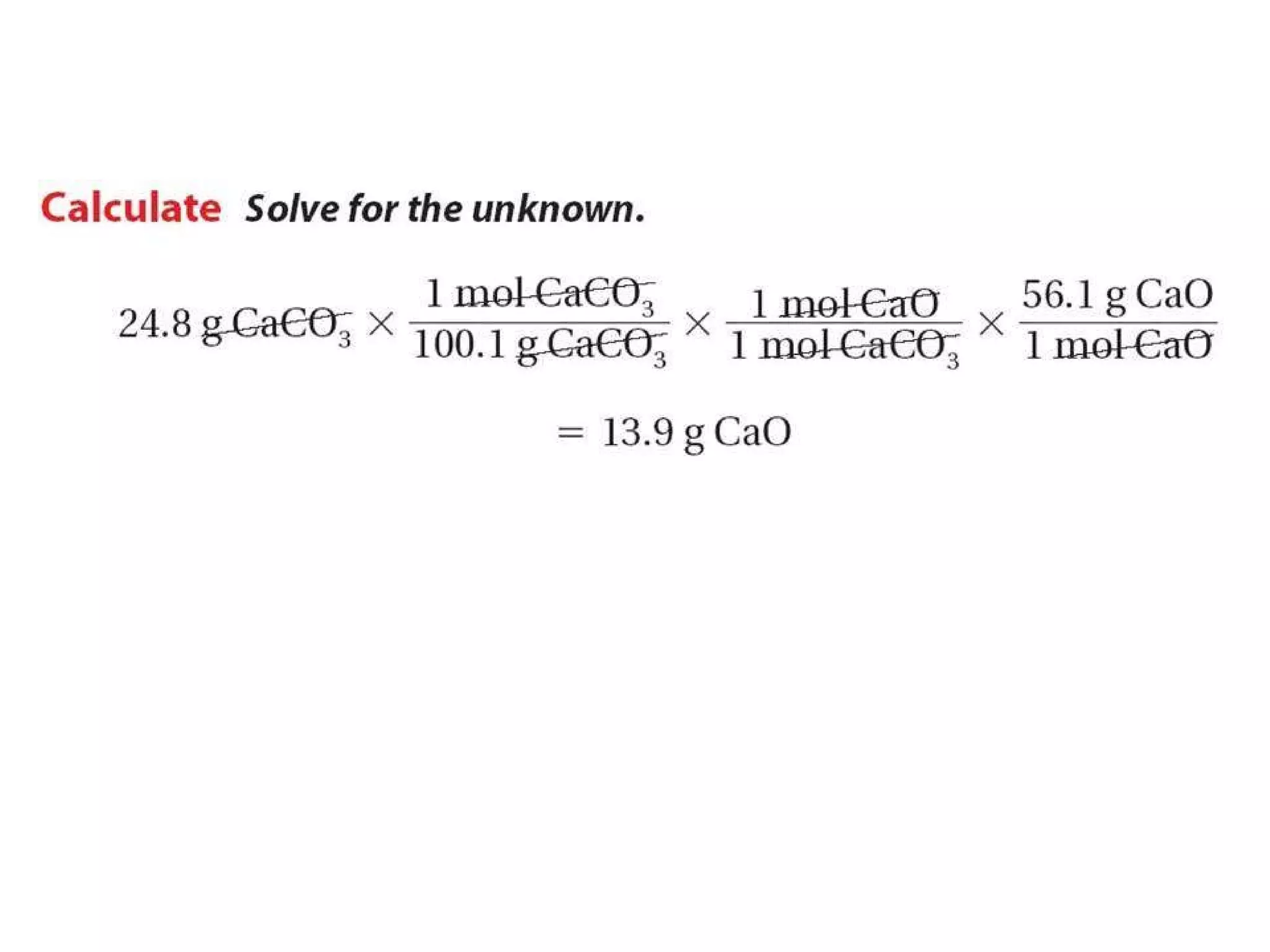
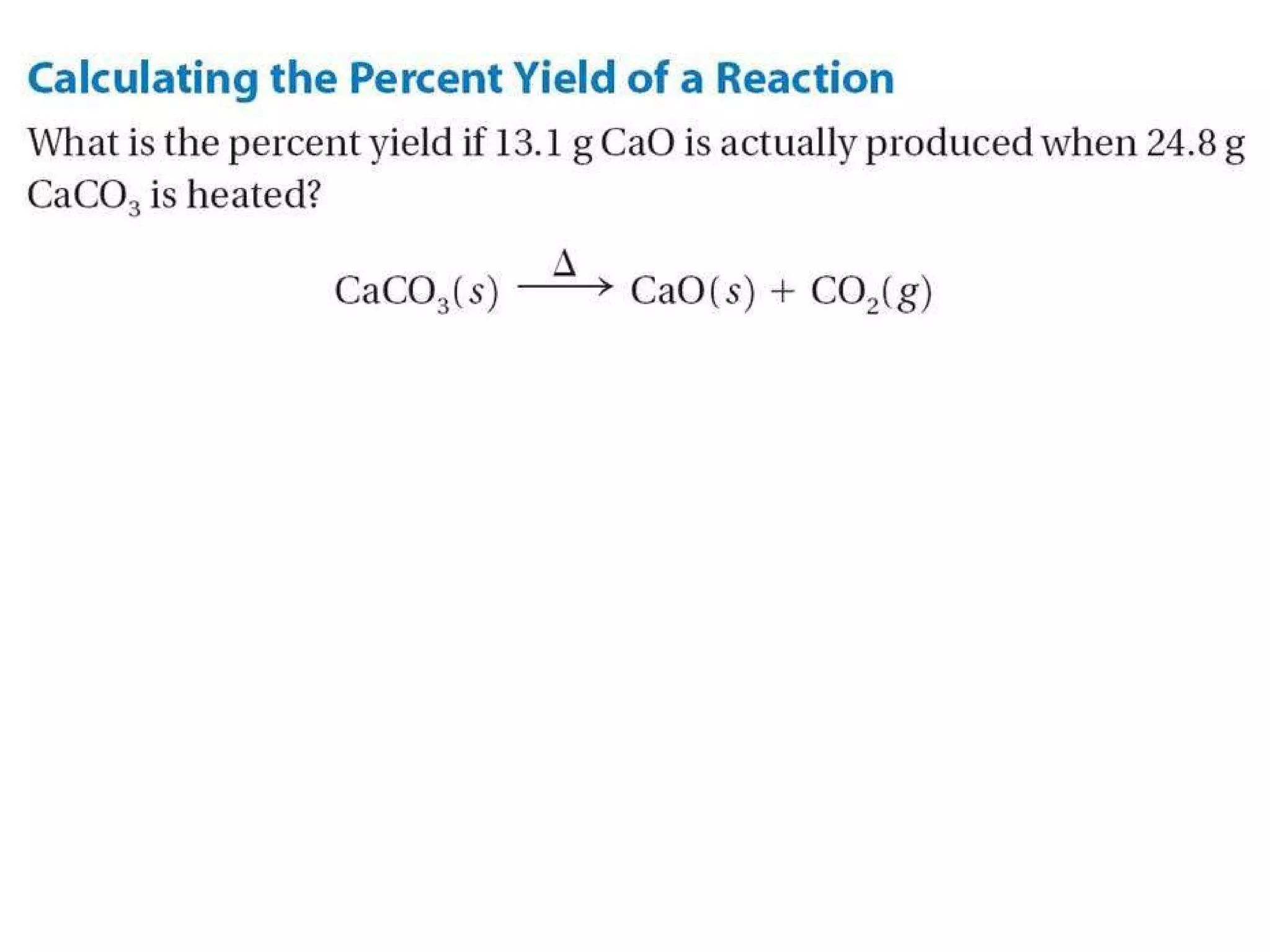
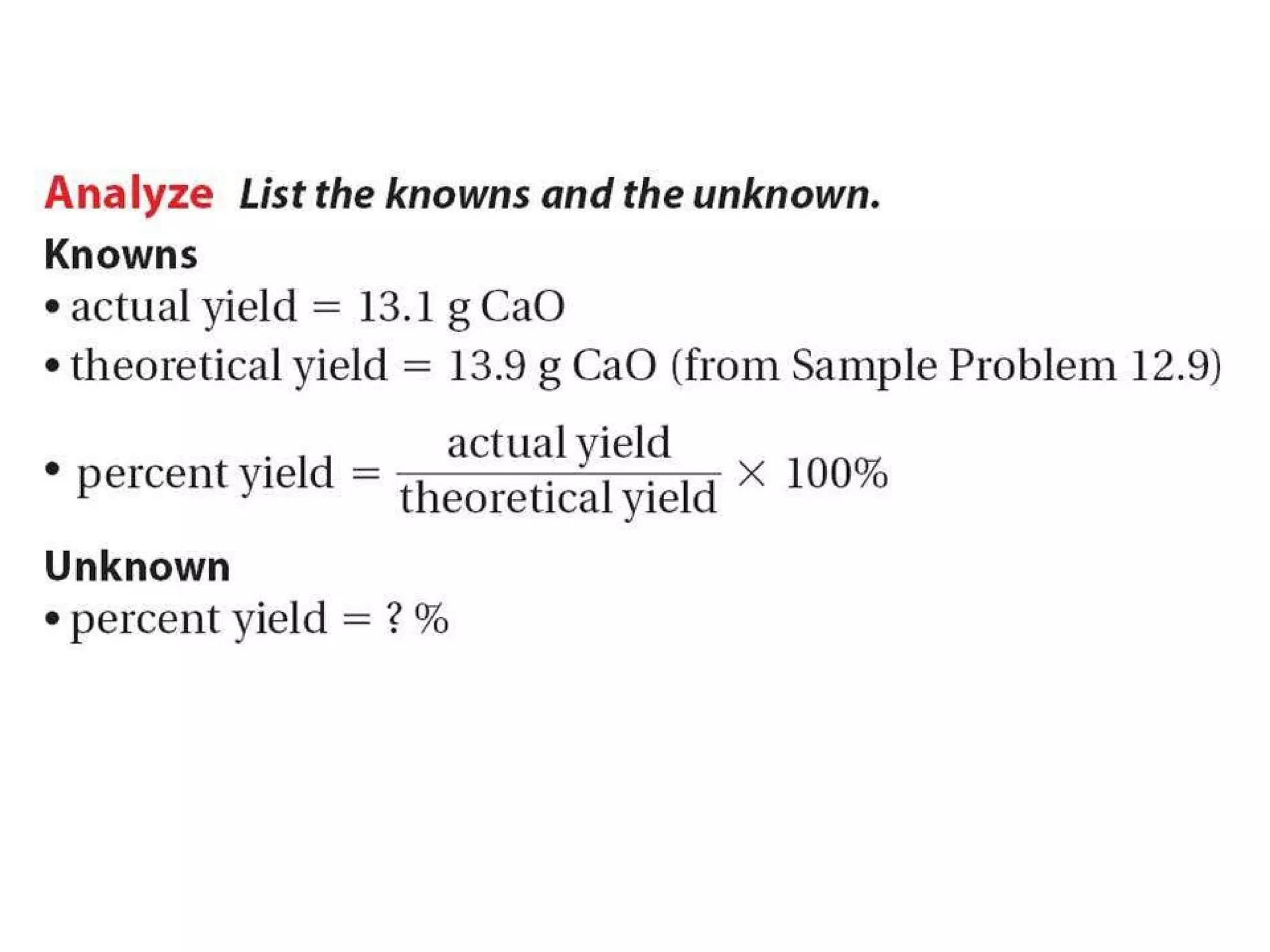
2) Theoretical yield refers to the maximum product that can form based on stoichiometry while actual yield is what is obtained in the lab.
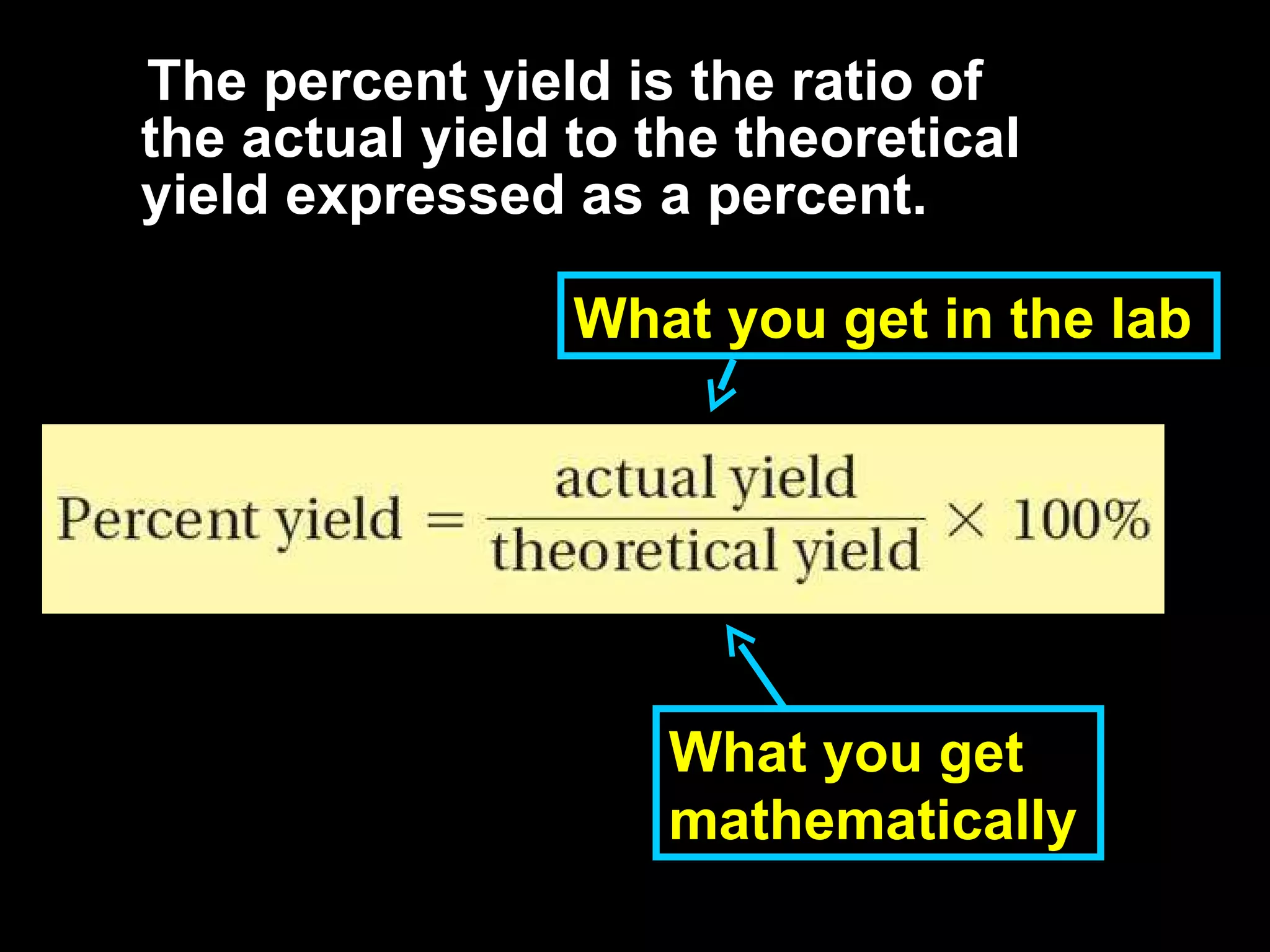
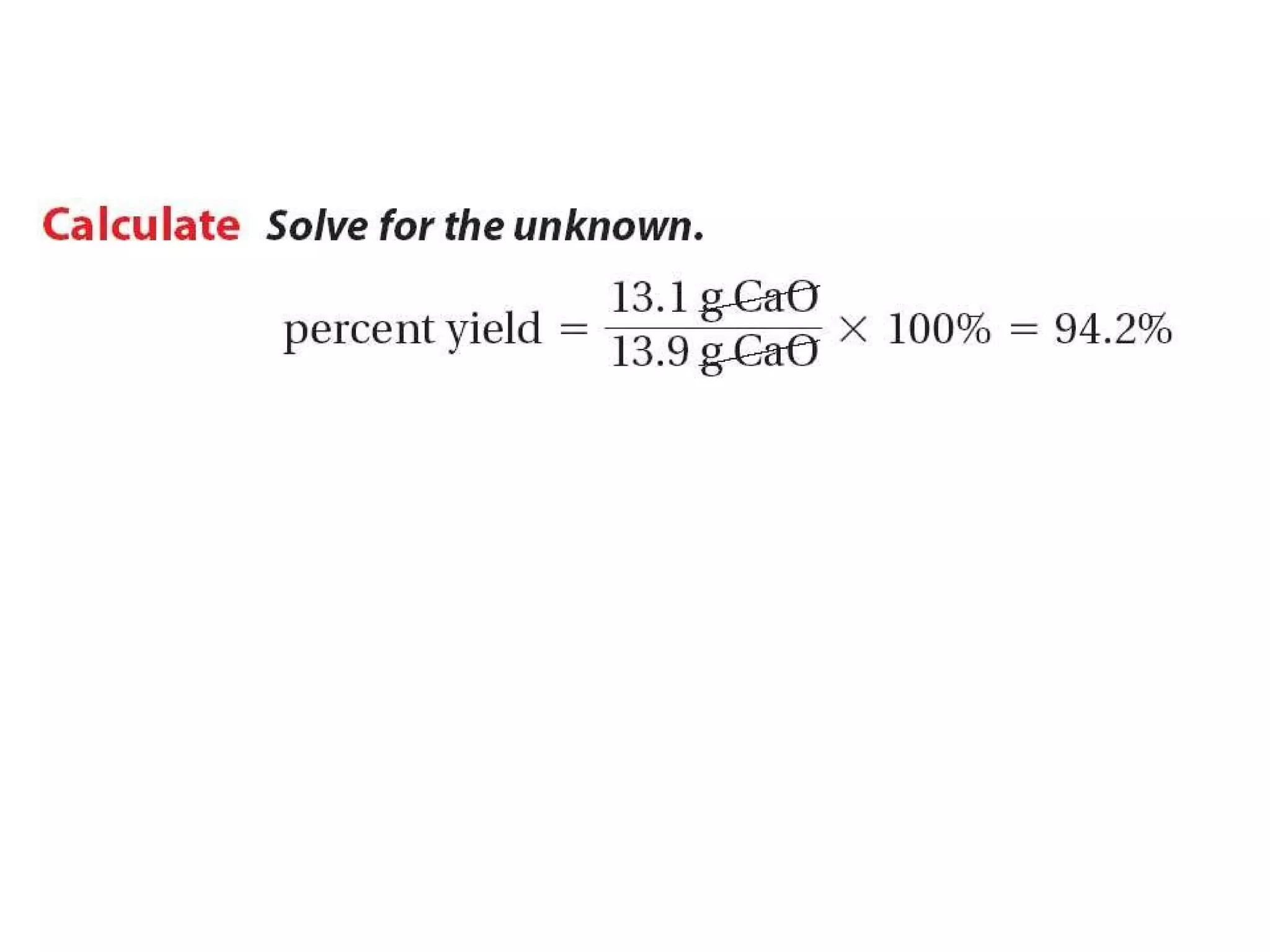
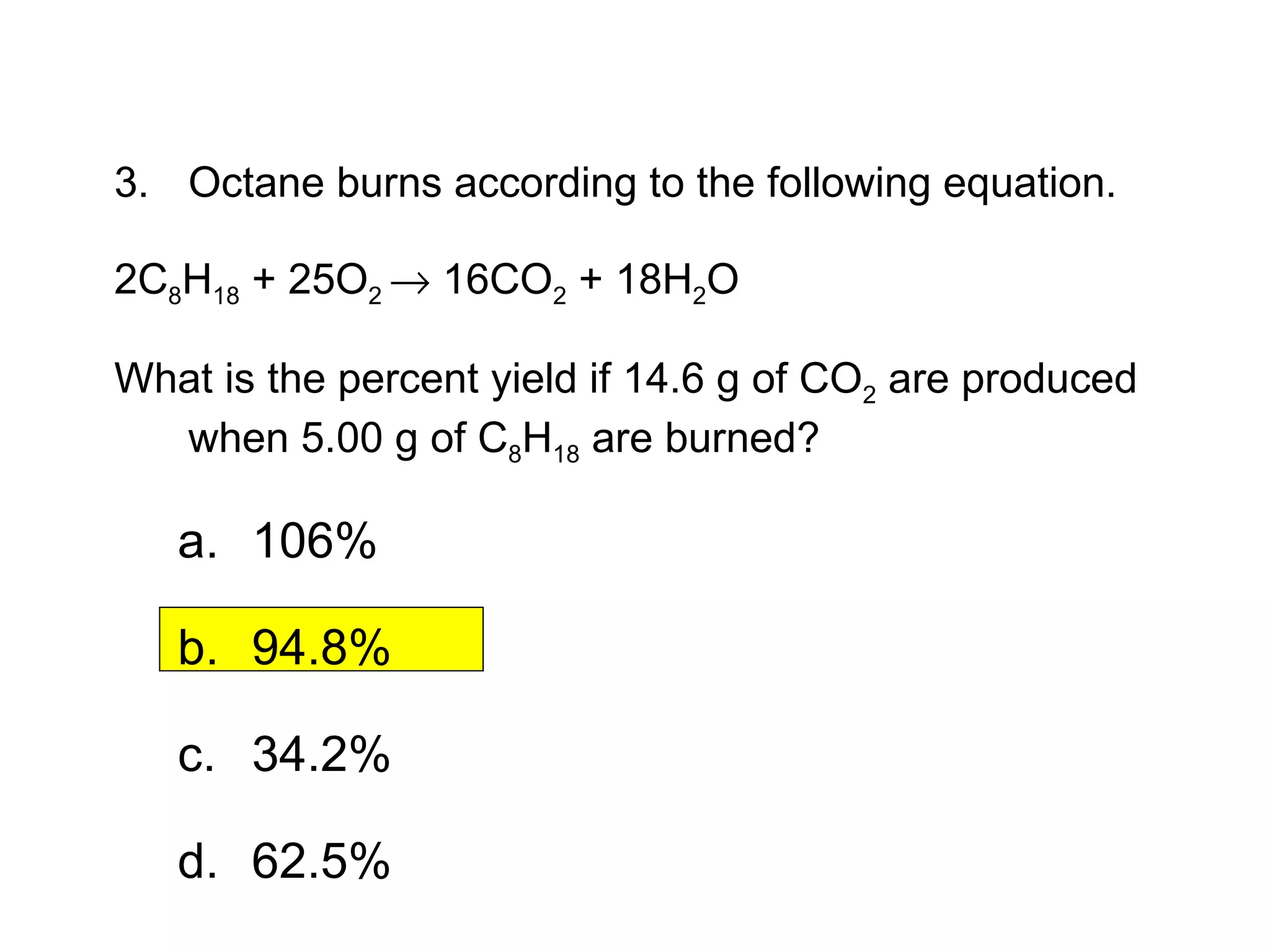
3) Percent yield compares the actual yield to the theoretical yield and indicates the reaction's efficiency. It is never higher than 100%.